Equipment
Barbecue lighter, Screwdriver, Handheld propane torch, Gloves, Tongs, Insulating pad or towel
Reagents
5lb slabs of dry ice (2), 30 g of Mg turnings
Presentation
Preparation
- Using a flathead screwdriver, gouge a hole in the middle of one of the slabs of dry ice. The hole should be a hemisphere, roughly 3-4 cm in diameter.
- Using a flat, polished metal surface, polish one side of each dry ice block until the two pieces fit together without a gap.
- Store the dry ice in an insulated container until the time for the presentation of the demonstration.
Demonstration
- Remove the indented piece of dry ice from the container and place it on a towel.
- Fill the cavity with magnesium turnings.
- Make sure that no combustible materials are near the apparatus.
- The next steps are best done with a partner. Light the magnesium turnings. This will take 15 seconds or so, since oxygen has been depleted from the air around the turnings and the magnesium is quite cold.
- While one person is lighting the magnesium, the other person should be ready to place the other piece of dry ice over the burning magnesium. This needs to be done quickly in order to minimize the reaction of magnesium with molecular oxygen which is a faster and more exothermic reaction.
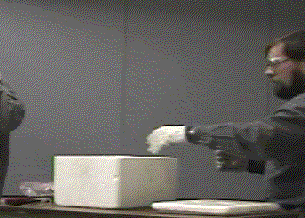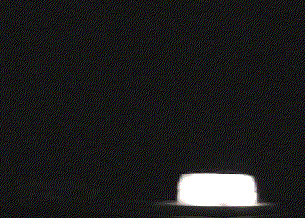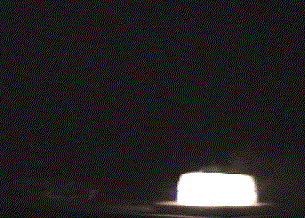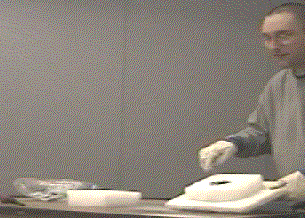
- Once the other piece of dry ice is in place, step back, dim the lights and watch the dry ice glow brilliantly white!
- Once the reaction has ceased to glow (3-5 minutes), remove the top piece of dry ice and show the white MgO. Using the tongs, the shell of the MgO ball can be cracked open to reveal a black powder which is elemental carbon.
Hazards
Work in a well-ventilated area. Do not look directly at burning magnesium. The reaction will release some fine particles of magnesium and magnesium oxide into the air. This can be harmful if inhaled in large quantities. Neither a carbon dioxide nor a water fire extinguisher will put out a magnesium fire. Should a fire result, the best thing to do is to let it burn out on its own, provided it is not growing out of control. In that case call the fire department.
Discussion
The white, flaky material is MgO while the black residue is mostly carbon with traces of Mg3N2. The reaction is broken down into elementary steps as shown below. The energies associated with each step were taken from one of two sources, steps 1-6 [3] and steps 7-9 [2].
Elementary Step Associated energy (kJ) 1. 2 [ Mg (s) ® Mg (l) ] 2 [132] 2. 2 [ Mg (l) ® Mg (g) ] 2 [9.037] 3. 2 [ Mg (g) ® Mg 1+ (g) + e -] 2 [737.3] 4. 2 [ Mg 1+ (g) ® Mg 2+ (g) + e -] 2 [1,449.8] 5. CO2 (g) ® C (s) + O2 (g) 392.0 6. O2 (g) ® 2 O (g) 497.31 7. 2 [ O (g) + e - ® O 1- (g) ] 2 [-141] 8. 2 [ O 1- (g) + e - ® O 2- (g) ] 2 [878] 9. 2 [Mg 2+ (g) + O 2- (g) ® MgO (s) 2 [-3916] Net Reaction: 2 Mg (s) + CO2 (g) ® 2 MgO (s) + C (s) net energy: -812
The dominant thermodynamic term that gives rise to the exothermicity of the reaction is the lattice energy, step 9. Because of the stability of magnesium oxide, carbon dioxide yields its oxygen to magnesium to form this product. Magnesium will react with other oxides. The key factor is how much energy is required to decompose the oxide as illustrated in step 5. Sand (SiO2) and water (H2O) will both react with magnesium under the proper conditions. Unlike the alkali metals, magnesium does not react violently with water at room temperature. The reason for this difference is that a layer of insoluble magnesium oxide forms around the magnesium which separates the remainder of the magnesium from the water. With sodium and potassium, the heat of the reaction is enough to melt the remaining metal, thus increasing the accessible surface area which vigorously accelerates the reaction. The bottom line is that magnesium will take oxygen from nearly any source to form the remarkably stable compound, magnesium oxide.
References
- B. Z. Shakhashiri, Chemical Demonstrations, A Handbook for Teachers of Chemistry, Wisconsin, 1989, Vol.1, p.90-92.
- Zumdahl, S. S.; Zumdahl, S. A., Chemistry, 5th ed.; Houghton Mifflin: Boston, 2000.
- Handbook of Chemistry and Physics, 54th ed.; CRC Press, Cleveland, OH, 1973-1974.