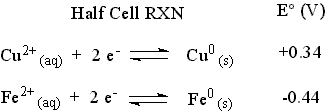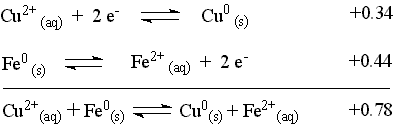Plating Copper onto Iron
Equipment
Piece of iron (strip, rod, etc.), 250-mL beaker.
Reagents
Just about any Cu2+ containing solution at a concentration of 0.1-1.0 M.
Presentation
- Pour 50-150 mL of the Cu2+ solution into the beaker.
- Dip the piece of iron into the Cu2+ solution. The copper will begin plating the iron immediately and within seconds the copper coating will be quite visible.
Hazards
Solutions of Cu2+ irritate eyes and may irritate skin.
Discussion
The standard reduction potentials for the two half-cells are given below.
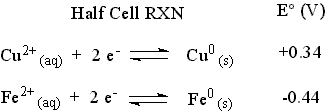
The reaction with the more positive E° proceeds as it is written (It is the cathodic reaction.). The reaction with the less positive (more negative) E° will be forced to proceed in the opposite direction as it is written (It is the anodic reaction.). Changing the direction of a reaction changes the sign of E° for the reaction. Add the two reaction according to the way they will be reacting and sum the appropriate E° values to produce the overall cell reaction and the cell potential.
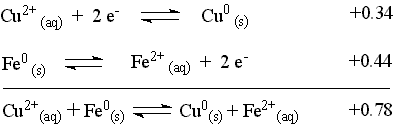
Physically, this says that the Cu2+ concentration will decrease as copper ions are reduced and deposited on the surface of the iron and the iron strip will be getting lighter as iron atoms are oxidized and leave the surface and enter the solution. If allowed to continue, the solution would lose the blue color from the Cu2+ and become a pale green from the Fe2+.
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 3/26/2000.
For questions or comments, contact
Joseph Franek.