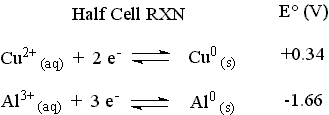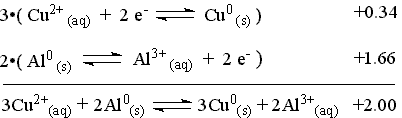Tearing an Aluminum Can in Half
Equipment
You will need an aluminum can, and a container large enough to sit the can in.
Reagents
Approximately 400-500 mL of 1 M CuCl2.
Presentation
- Rinse the aluminum can.
- Score the inside of the can about half way up completely around the circumference of the can. This removes the plastic lining and exposes the bare aluminum.
- Place the can in the protective container.
- Pour enough Cu2+ solution into the can so that the solution level is above the score mark.
- Wait approximately 1-3 minutes.
- Pour the solution out of the can.
- Grip each end of the can and simultaneously twist and pull. The can will tear apart easily along the score.
- You can play a trick by using a scored can and an unscored can and choosing two volunteers from the audience (one clearly stronger than the other). After finishing step 6, give the unscored can to the stronger volunteer. The weaker volunteer will tear apart their can with ease.
Hazards
Solutions of CuCl2 irritate eyes and may irritate skin.
Discussion
The standard reduction potentials for the two half-cells are given below.
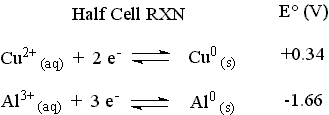
The reaction with the more positive E° proceeds as it is written (It is the cathodic reaction.). The reaction with the less positive (more negative) E° will be forced to proceed in the opposite direction as it is written (It is the anodic reaction.). Changing the direction of a reaction changes the sign of E° for the reaction.
Add the two reaction according to the way they will be reacting and sum the appropriate E° value to produce the overall cell reaction and the cell potential.
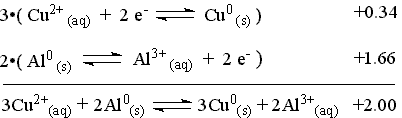
This reaction can only take place once the aluminum metal is exposed from behind the protective plastic coating.
References
- Lee R. Summerlin, James L. Ealy, Chemical Demonstrations, A Sourcebook for Teachers, Washington D.C., American Chemical Society, Second Edition, 1988, Vol. 1, p.152-153.
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 3/26/2000.
For questions or comments, contact
Joseph Franek.