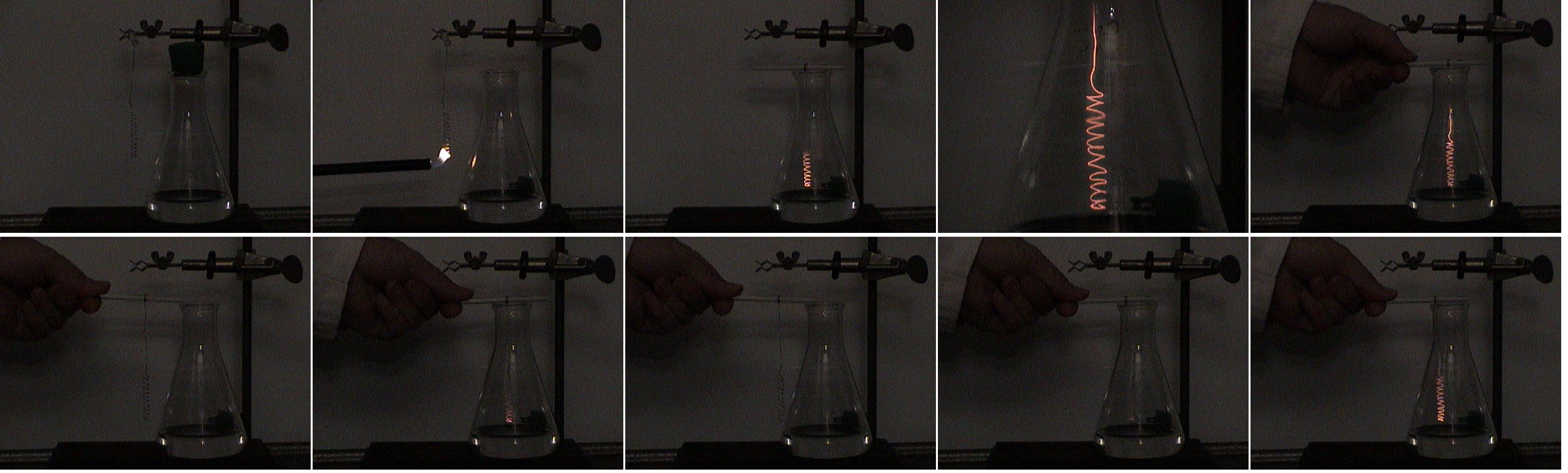
Equipment
250-mL Erlenmyer flask, rubber stopper for flask, barbecue lighter, rod to support wire across the top of the flask, coiled platinum wire. The coil should be approximately 7 mm in diameter and loose enough that the adjacent coils so not touch.
Reagents
25-75 mL of concentrated aqueous ammonia solution.
Presentation
- Pour the ammonia solution into the flask and stopper it.
- Attach wire to its support.
- Adjust the length so that the end of the platinum is 1-3 cm above the surface of the ammonia solution.
- Unstopper the flask.
- Heat the end of the platinum wire until it begins to glow red.
- Quickly, put the wire down into the flask.
- The wire will glow brightly.
- The wire may be removed from the flask and it will cease to glow.
- If the wire is returned to the flask before it gets too cool, it will again glow brightly. This may be repeated many times.
Hazards
Concentrated aqueous ammonia can cause burns and is irritating to the skin, eyes, and respiratory tract. Nitrogen dioxide is an extremely toxic gas. It is irritating to the respiratory tract.
Discussion
Ammonia is catalytically oxidized at the platinum surface according to the following reaction.The reaction is exothermic and as written releases 920 kJ of energy. A second reaction proceeds automatically from the first reaction. 5 NH3 (g) + 5 O 2 (g) ® 4 NO (g) + 6 H2O (g)The second reaction is also exothermic and as written releases 112 kJ of energy. These two reactions taken together make up two thirds of the Ostwald process for the synthesis of nitric acid. If the NO2 (g) were put into contact with liquid water, nitric acid and NO (g) would be produced. The initial heating of the wire with the lighter provides the actvation energy for the reaction. Once the reaction begins, the released energy will provide more than enough activation energy for subsequent reactions to occur. An interesting variation of this demonstration is to substitute a thin copper wire, approximately 0.2 mm in diameter for the platimun wire (2). The heat from the reaction is sufficient to melt the copper wire. The molten copper will spatter and make a spectacular effect. Additionally, some of the copper will oxidize and turn the ammonia solution blue by forming a complex ion between the Cu 2+ ion and the ammonia [Cu(NH 3) 4] 2+. This variation does not work as consistently well as the platinum version does. 2 NO (g) + O 2 (g) ® 2 NO2 (g)
References
- B. Z. Shakhashiri, Chemical Demonstrations, A Handbook for Teachers of Chemistry, Wisconsin, 1989, Vol.2, p.214-215
- Gilbert, Alyea, Dutton, Dreisbach, Tested Demonstrations in Chemistry, Published by arrangement with the Journal of Chemical Education, 1994, Vol. I, p.I-35