Catalytic Decomposition of Hydrogen Peroxide
by Iodide
"Elephant's Toothpaste"
Large graduated cylinder (500 mL) or 2 liter soda bottle, goggles, plastic
tray or sheet
1. Place graduated cylinder or soda bottle in a plastic tray or on a large
sheet of plastic.
2. Pour ~50 mL of 30% hydrogen peroxide into the cylinder or bottle.
3. Add a squirt of dishwashing detergent; agitate slightly.
4. Add ~10 mL of potassium iodide solution OR 1/4 spoonful of solid potassium
iodide. (Note: The reaction is much faster with the KI solution.) Step back
quickly after adding the potassium iodide.
- Hazards
30% hydrogen peroxide is a strong oxidizing agent; contact with eyes and
skin should be avoided. In case of contact, flush with water for at least
15 minutes. Get medical attention if eyes are affected. Also avoid contact
of hydrogen peroxide and combustible materials. 30% hydrogen peroxide must
be stored in its original container.
- Discussion
Oxygen is a colorless, odorless gas at room temperature and atmospheric
pressure. It is formed here by the catalytic decomposition of hydrogen peroxide
by the iodide ion.
H2O2 (aq) + I-(aq) 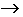 OI-(aq)
+ H2O(l) Step 1
OI-(aq)
+ H2O(l) Step 1
H2O2 (aq) + OI-(aq) 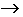 I-(aq)
+ H2O(l) + O 2(g) Step 2
I-(aq)
+ H2O(l) + O 2(g) Step 2
2 H2O2 (aq) 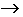 2
H2O(l) + O 2(g) Overall
2
H2O(l) + O 2(g) Overall
The first step is the rate limiting step of the reaction. The oxygen that
is produced causes the dishwashing detergent to foam; the foam will shoot
out of the container! Note that the iodide does not appear in the overall
reaction. The overall reaction is exothermic; the heat produced is enough
to slightly shrink the plastic of the two liter bottle.
For an interesting twist, put a small amount of food coloring (~5-10 drops)
in a strip along the wall of the soda bottle or graduated cylinder (more
dramatic if done with graduated cylinder. . The resulting foam will have
a stronger resemblance to toothpaste.

- L. R. Summerlin and J. L. Ealy, Jr. Chemical Demonstrations, A Sourcebook
for Teachers, Washington D. C., 1985, p.71.
Request a demonstration
The University of Minnesota is an equal opportunity educator
and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 06/16/2004.
For questions or comments, contact Joseph
Franek.
 OI-(aq)
+ H2O(l) Step 1
OI-(aq)
+ H2O(l) Step 1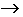 I-(aq)
+ H2O(l) + O 2(g) Step 2
I-(aq)
+ H2O(l) + O 2(g) Step 2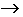 2
H2O(l) + O 2(g) Overall
2
H2O(l) + O 2(g) Overall