Structural Dynamics and Topology of Phospholamban
in Oriented Lipid Bilayers using Multi-Dimensional Solid-State NMR
Recently, Gianluigi Veglia and
his research group published a hot paper in Biochemistry
characterizing the
structural dynamics and topology of phospholamban (PLN) in oriented lipid
bilayers using solid-state nuclear magnetic resonance spectroscopy [Traaseth et
al. 2006, Biochemistry 45, 13827-34].
PLN is a single-pass
transmembrane protein that regulates heart muscle contraction and relaxation by
reversible inhibition of Ca-ATPase, a large membrane enzyme responsible for Ca2+
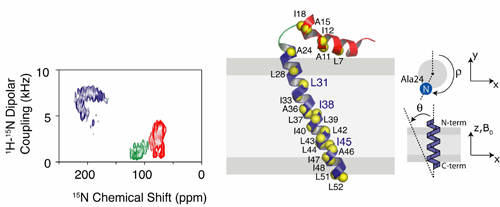
flow into the sarcoplasmic reticulum. In this paper, two-dimensional PISEMA (an
experiment correlating 1H-15N dipolar coupling with the
anisotropic 15N chemical shift) carried out in DOPC/DOPE mixed lipid
bilayers fully reveal the tilt and rotation angles of the transmembrane and
cytoplasmic domains of PLN with respect to the membrane bilayer normal. Additionally,
the research also revealed dynamic information. Specifically, PLN undergoes
fast long-axial rotational diffusion about the bilayer normal with the
cytoplasmic domain undergoing this motion and other complex dynamics. While
some of this dynamics was revealed by previous solution nuclear magnetic
resonance relaxation studies in detergent micelles, these measurements in
anisotropic native lipid environment reveal new dynamic features encoded in the
free protein that might be crucial for Ca-ATPase recognition and the inhibitory
mechanism.
|