Structural and Functional Model of the Rieske Dioxygenases
Professor Larry
Que and his research group:
The
initial step in remediation of aromatic compounds in the environment involves
the cis-dihydroxylation of an arene
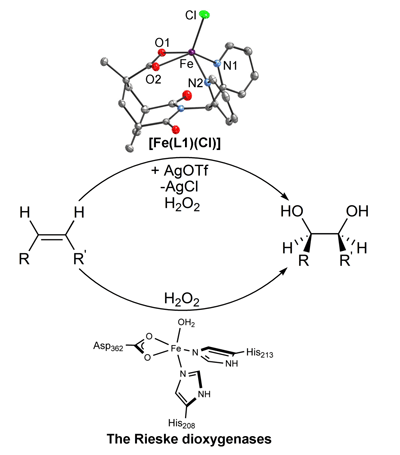
double bond. This unusual reaction is catalyzed by the Rieske dioxygenases,
bacterial enzymes that have mononuclear iron active sites with a 2-His-1-carboxylate
facial triad. In an article to appear in Angew. Chem. Int. Ed.,
graduate student Paul Oldenburg and postdoctoral associates Chun-Yen Ke and
A. Alex Tipton in the group of Professor Larry
Que report the structure and
characterization of [Fe(L1)(Cl)], a complex that serves as the first structural and functional model of the Rieske dioxygenases. The polydentate
L1 ligand provides two pyridine donors and a bidentate carboxylate, closely
mimicking the iron environment in the Rieske dioxygenase active site. [Fe(L1)(Cl)]
promotes olefin epoxidation with H2O2 as oxidant but
effects instead, after dechlorination by treatment with AgOTf, the desired cis-dihydroxylation
of olefins. This contrasting behavior emphasizes the requirement for two
available cis sites for dihydroxylation.
Isotopic labeling studies suggest that the activation of H2O2 at
the iron center involves formation of a cis HO-Fe(V)=O oxidant.
|