12/28/2007
The first
synthetic complex with a [FeIV2(μ-O)2] diamond core
Recent research from the group of Professor Lawrence Que.
In
a paper that appears in the December 26, 2007 issue of Proceedings of
the National Academy of Sciences USA (Proc. Natl. Acad. Sci. USA 2007, 104, 20713-20718), Prof. Larry Que,
postdoctoral associate Genqiang Xue, and coworkers report the characterization
of the first example of a synthetic diiron(IV) complex with an [Fe2(μ-O)2] diamond core. This synthetic
precedent lends credence to the postulation of such a core structure for
intermediate Q, the key species responsible for the hydroxylation
of methane in the catalytic cycle of soluble methane monooxygenase (MMO)
[1]. The first crystal structure of an iron(IV)-oxo complex was reported
only in 2003 [2], and the title complex may be considered as a head-to-tail
dimer of such complexes.
To
achieve this goal, Dr. Xue and graduate student Dong Wang performed low temperature
bulk electrolysis on [FeIIIFeIV(μ-O)2(L)2]3+ (1),
a complex previously characterized crystallographically to have the [Fe2(μ-O)2]
diamond core structure but with an iron(III)iron(IV) oxidation state [3].
One-electron oxidation of 1 at 0.9 V vs ferrocene resulted in the near-quantitative
formation of new complex 2, as determined by coulometry. Figure 1 shows the
conversion of 1 to 2 as followed by spectroelectrochemistry with the appearance
of at least two isosbestic points. Further characterization of 2 established the presence of the
[FeIV2(μ-O)2] diamond core, specifically
by the use of Mössbauer spectroscopy (by graduate student Raymond De Hont
and Prof. Eckard Münck of Carnegie Mellon University), resonance Raman spectroscopy
(by Drs. Xue and Xiaopeng Shan) and EXAFS analysis (by Dr. Adam Fiedler).
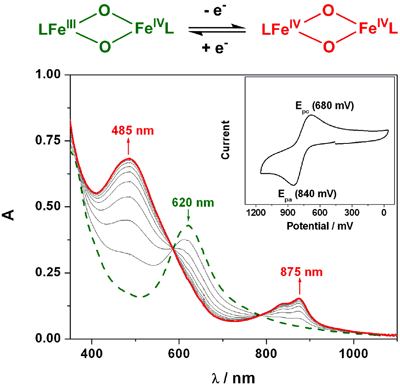
Figure
1. Electrochemical oxidation of an [FeIIIFeIV(μ-O)2]3+ precursor (green
dashed line) to the [FeIVFeIV(μ-O)2]4+ complex (red
solid line).
With
the diiron(IV) complex in hand, we have an unprecedented opportunity to compare
the reactivity properties of three different iron(IV)-oxo core units that
are supported by the same ligand, namely [FeIIIFeIV(μ-O)2]
(1), [FeIV2(μ-O)2] (2) and terminal FeIV=O (3)
units. Kinetic results showed that the rates of
hydrogen-atom abstraction increased in the order: 1 < 2 < 3,
with 3 100-fold more reactive than 2. This surprising observation raises the possibility
that the [FeIV2(μ-O)2]
diamond core of Q may need to convert
to a ring-opened form to break the strong C-H bond of methane.
[1]
Shu, L., Nesheim, J. C., Kauffmann, K., Münck, E., Lipscomb, J. D., & Que,
L., Jr. (1997) Science 275, 515-518.
[2]
Rohde, J.-U., In, J.-H., Lim, M. H., Brennessel, W. W., Bukowski, M. R.,
Stubna, A., Münck, E., Nam, W., & Que, L., Jr. (2003) Science 299, 1037-1039.
[3]
Hsu, H.-F., Dong, Y., Shu, L., Young, V. G., Jr., & Que, L., Jr. (1999) J.
Am. Chem. Soc. 121, 5230-5237.
|