Insight into the role of Mg(II) in hammerhead ribozyme catalysis
from X-ray crystallography and molecular dynamics simulation
Recent Research from Professor Darrin York and his
research group.
The hammerhead ribozyme is an archetype system
to study RNA catalysis. A detailed understanding of the hammerhead
mechanism provides insight into the inner workings of more complex
cellular catalytic RNA machinery such as the ribosome, and ultimately
may aid the rational design of new medical therapies and biotechnology.
Despite a tremendous amount of experimental
and theoretical effort, the details of the hammerhead ribozyme mechanism have been elusive. In particular, one
of the main puzzles involves the apparent inconsistency between the
interpretation of thio effect experiments and mutational
data with available crystallographic structural information of the
minimal hammerhead sequence. Results from the biochemical experiments
suggest that a pH-dependent conformational change, inconsistent with
crystallographic data, must precede or be concomitant with the catalytic
chemical step. This includes a possible metal ion bridge between the
A9 and scissile phosphates that in previous crystal structures were
~20 Å apart. Moreover, the function of the 2’OH group of G8 remains
unclear.
Recently, the research groups of Professor Darrin York,
scientist Tai-Sung
Lee and postdoc Carlos Silva-Lopez of the Department of Chemistry,
in collaboration with Professor Bill Scott and
his graduate student Monika Martick of the
Department of Chemistry, UCSC, have performed molecular simulations
that probe the conformational events and metal ion binding that leads
to ribozyme catalysis. A series of 12 ns molecular dynamics (MD) simulations
of the reactant state (with and without a Mg(II) ion), early and late
transition state mimics are presented based on a recent crystal structure
of a full-length hammerhead RNA reported by Martick and Scott (Fig.
1).
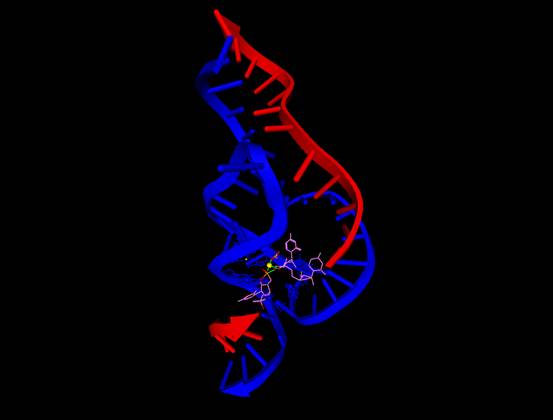
Figure 1. The full-length hammerhead
RNA reported by Martick and Scott [Cell 126, 309
(2006)].
Their simulation results support a catalytically
active conformation with a Mg(II) ion bridging the A9 and scissile
phosphates (Fig 2). In the reactant state, the Mg(II) spends significant
time closely associated with the 2’OH of G8, but remains fairly
distant from the leaving group O5’ position. In the early TS
mimic simulation, where the nucleophilic O2’ and
leaving group O5’ are equidistant from the phosphorus, the Mg(II)
ion remains tightly coordinated to the 2’OH of G8, but is positioned
closer to the O5’ leaving group, stabilizing the accumulating
charge. In the late TS mimic simulation, the coordination around the
bridging Mg(II) ion undergoes a transition whereby the coordination
with the 2’OH of G8 is replace by the leaving group O5’ that
has developed significant charge. At the same time, the 2’OH
of G8 forms a hydrogen bond with the leaving group O5’ and is
positioned to act as a general acid catalyst.
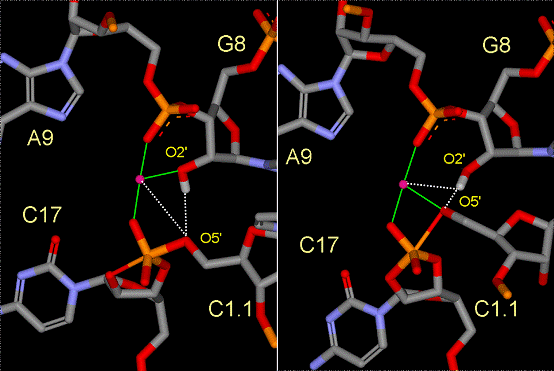
Figure
2. Snapshots of the active site from the early TS mimic (left)
and late TS mimic (right) simulations depicting the Mg(II)
ion direct coordination (green lines) and key hydrogen bonds
and indirect Mg(II) coordination (dotted lines). For clarity,
the water molecules are not shown.
This work represents the first reported simulations of the full-length
hammerhead structure (Fig 2) and TS mimics, and provides direct evidence
for the possible role of a bridging Mg(II) ion in catalysis that is
consistent with both crystallographic and biochemical data. It has
been published on-line in J.
Chem. Theory Comput.
|