07/24/2007
Computer Simulations Show that the Hydrogen Radical
Transfer Reaction Catalyzed by Methylmalonyl-CoA Mutase and Coenzyme B 12 is Dominated by Extreme
Quantum Mechanical Tunneling
Recent Research from the group of Professor
Donald Truhlar.
A subclass of the coenzyme B12-dependent
isomerases catalyze chemically challenging carbon-skeleton rearrangements.
For example, methylmalonyl-CoenzymeA mutase (MMCM), which catalyzes the reversible
isomerization of methylmalonyl to succinyl in both human and bacteria. This
reaction represents an intermediate step in the catabolism of the odd‑chain
fatty acids, branched-chain amino acids and cholesterol, and its impairment
results in methylmalonic aciduria.
Coenzyme B12 is
an extensively modified porphyrin whose structure was determined by X-ray
crystallography by Hodgkin in 1961.

The structure revealed, unexpectedly at the time,
a cobalt-carbon bond to the deoxyadenosyl component of B12.
Homolytic rupture of this bond is a key step in the catalyzed rearrangement,
but atomistic details of the mechanism have remained elusive. Recent advances
in computational enzyme kinetics have allowed for simulations that help to
elucidate the mechanisms of enzyme-catalyzed reactions, and Agnieszka
Dybala-Defratyka and Piotr Paneth of the Technical University of Lodz
in Poland, Ruma Banerjee of the University of Nebraska,
and Donald G. Truhlar of the University of Minnesota have now uncovered
the atomistic details of the process and have shown that the experimental
results, from the laboratory of Professor Banerjee, can be explained by extreme
quantum mechanical tunneling. The results are published in the June 26 issue
of the Proceedings of the National Academy of Sciences.
The bacterial MMCM studied in this
work is a heterodimer (with units called α and β)
as shown in this figure:
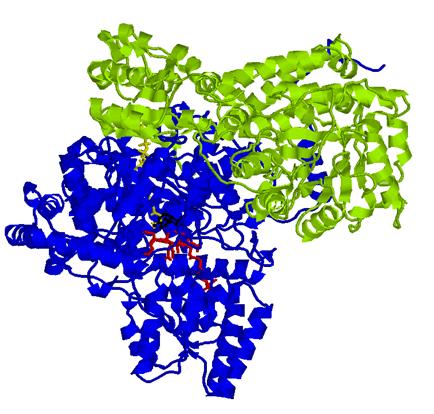
The α subunit (blue)
contains the active site of the enzyme in which the cobalamin moiety of conenzyme
B12 is in red, the substrate
methylmalonyl-CoenzymeA is in yellow, and the 5'-deoxyadenosyl group of B12 is
in black; the β subunit of the dimer
is in green.
The key reaction is a hydrogen radical transfer
as illustrated in the following scheme:
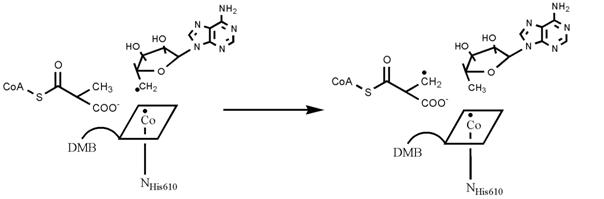
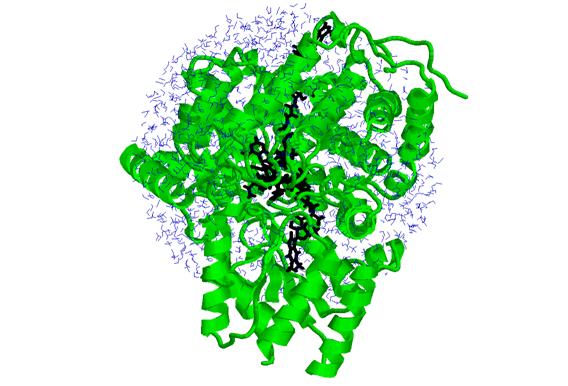
The atoms involved
in the simulation are shown in the structure below, which contains 14878
atoms. This includes 672 amino acids (in green ribbons), 1388 water molecules
(in blue), and 299 atoms of the active site residues (in black).
The simulations are based on transition state theory,
which identifies the dynamical bottlenecks, called transition states, that
represent points of no return along the reaction path. To a very good approximation,
the rate of forming the dynamical bottleneck species is equal to the overall
reaction rate. Attainment of the transition state may to likened to passing
over a "mountain" pass or barrier; the bottleneck corresponds to
the barrier top. In order to calculate quantitative reaction rate constants,
eight transition state configurations were chosen, and an ensemble of eight
reaction paths passing through these transition states was calculated. The
total transmission coefficient, which is the factor by which tunneling increases
the rate constant, was is obtained by averaging the dynamics over these eight
paths.
The primary experimental observable is the kinetic
isotope effect, which is the ratio of the reaction rate for the system shown
in the scheme above to the reaction rate when the CH3 group
that loses a hydrogen is replaced by CD3,
where H denotes protium (the lightest isotope of hydrogen) and D denotes
deuterium (a heavy isotope of hydrogen). Protium atom is lighter than deuterium
and has a larger quantum mechanical zero point energy and a larger probability
of tunneling. Both of these differences contribute to the reaction rate
being higher for H than for D. In fact the experimental kinetic isotope effect
is very dramatic—it is 49. That is, the rate constant for the CH3 case
is 49 times larger for hydrogen transfer than for deuterium transfer. However,
the calculations show that in the absence of tunneling, the kinetic isotope
effect would be only 14. When tunneling is included the calculated kinetic
isotope effect is increased to 51, in excellent agreement with experiment. This
provide confidence in the detailed dynamic picture of the reactive event
that is afforded by the computer simulation.
Tunneling corresponds to the system passing through the "mountain" rather
than over the "mountain" pass. This could not occur for
macroscopic objects, but because the H and D are very light they are described
by the laws of quantum mechanics rather than the laws that govern the behavior
of macroscopic objects. The simulation shows that the H reaction is speeded
up by a factor of 93 due to tunneling, and the D reaction is speeded up by
a factor of 26. A factor of 93 means that only 1% of the reaction proceeds
in the classical way, by passing over the barrier. When tunneling dominates
a reaction to this extent, it is called extreme tunneling.
Because
the final results of the quantum mechanical atomistic simulation agree
with experiment so well, the researchers were able to analyze them to better
understand the nature of the tunneling events. They found that the tunneling
of H or D is strongly coupled to motion of the other atoms in the active
site of the enzyme, and they were able to identify the geometrical configuration
at the critical configuration of the tunneling process. It is very gratifying
that 46 years after the structure of coenzyme B12 was
determined, the atomistic details of this biologically important reaction
that it catalyzes has now been elucidated.
This
work was supported in part by Polish State Committee for Scientific Research,
the Fogarty International Research Collaboration Award, the National Institutes
of Health, the National Science Foundation, and the University of Minnesota Supercomputing Institute.
|