05/24/2007
Receptor-Based Chemical
Sensors With Three-Dimensionally Ordered Macroporous Carbon Contacts
Recent Research from Professor Philippe Buhlmann and their research group.
In
a collaboration between the research groups of Professors Philippe Buhlmann
and Andreas Stein, ion-selective electrodes (ISEs) with three-dimensionally
ordered macroporous (3DOM) carbon as novel solid contact were developed
and found to exhibit much higher signal stability than previously reported
solid-contacted ISEs.
Chemical
sensors based on receptor-doped polymeric membranes have been developed
for over 60 analytes. They are routinely used in clinical chemistry for
well over a billion measurements per year. However,
to be suitable as implantable sensors for human health monitoring, receptor-based
ISEs need to be small and robust, and the sensor outputs needs to be stable
over long periods. In conventional ISEs, the sensing membrane is interposed
between the sample and an inner filling solution, but unfortunately use
of such inner filling solutions impedes miniaturization. Self-assembled
monolayers and conducting polymers have been used to replace the inner
filling solution, but the low redox capacitance, and pH and light sensitivity
limit the stability of the resulting sensors.
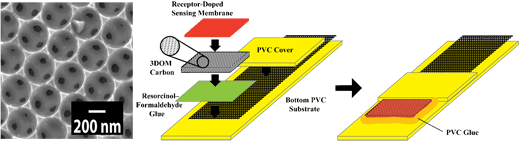
Figure 1. Left: SEM image of 3DOM carbon. Right:
Schematic structure of 3DOM carbon-contacted ISE.
For
this project, 3DOM carbon substrates are synthesized by graduate student
Melissa Fierke from the Stein group (Figure 1, left). Due to the well-interconnected
pore and wall structures with wall thicknesses of a few tens of nanometers,
3DOM carbon provides high ionic conductivity and electrical conductivity.
Graduate student Chun-Ze Lai from the Buhlmann group uses the 3DOM carbon
substrates to prepare and characterize chemical sensors (Figure 1, right)
The
theoretical ionic and redox responses of receptor-based potassium-selective
electrodes confirm the suitability of 3DOM carbon as solid contact. Moreover,
the 3DOM carbon-contacted electrodes exhibit unprecedented potential stability.
The potential drift for freshly prepared electrodes is only 11.7 ± 1.0 uV/h and does not increase over
one month while in contact with aqueous sample. Moreover, the electrodes
shows good resistance to the interference from O2 and light.
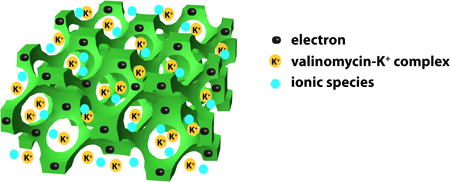
Figure 2. Schematic
bicontinuous electron- and ion-conducting structure of the interlayer
of doped 3DOM carbon.
The
high stability of the 3DOM carbon-contacted SC-ISEs is related to the bicontinuous
electron- and ion-conducting structure of the interlayer. On one hand,
the well-interconnected wall structure of the 3DOM carbon provides a continuous
pathway for electron conduction. On the other hand, ionic conductivity
is provided by a continuous network of interconnected pores that are filled
with the ionophore-doped solvent polymeric phase.
The
excellent potential stability makes 3DOM carbon-contacted ISEs very promising
for miniaturization and implantation. This work has been published on-line
on May 18 in Analytical Chemistry
|