11/7/2007
A Copper-Oxo Intermediate from O2?
Recent research from the groups of Professors William
B. Tolman and Christopher
J. Cramer.
Understanding the properties of synthetic copper-oxygen
intermediates is important for evaluating mechanisms of oxidation by enzymes
and other catalysts. A growing class of reactive copper-oxygen complexes
have been isolated or identified spectroscopically to date, including well-studied
examples with [Cu2(O2)]2+, [Cu2(m-O)2]2+,
or [CuO2]+ cores. While mononuclear copper-oxo species
([CuII-O-· « CuIII=O2-]+)
have been proposed as possible reactive intermediates in catalysis by copper
enzymes, they have only been observed in the gas phase and are less well
understood. In contrast, several routes have been developed to access the
closely related [FeIV=O]2+ moieties, of which one (found
in enzymes and model systems) involves the O2-induced decarboxylation
of an a-ketocarboxylate ligand. Drawing
an analogy to this pathway, graduate student Sungjun Hong working in the
laboratory of Professor William Tolman prepared
CuI-a-ketocarboxylate
complexes with bidentate N,N-donor
ligand sets containing an arene substituent and showed that upon reaction
with O2, decarboxylation and arene hydroxylation occurred (Figure).
These results have been rationalized through theoretical calculations performed
by postdoctoral associate Dr. Stefan M. Huber, Professor Christopher
Cramer, and Professor Laura
Gagliardi (University of Geneva), the results of which support two different
hydroxylation pathways that involve novel [CuI-OOC(O)R] and [CuII-O-· « CuIII=O2-]+ species
(the latter is shown in the Figure). These findings illustrate a new pathway
for the generation of novel copper-oxygen intermediates relevant to oxidation
catalysis.
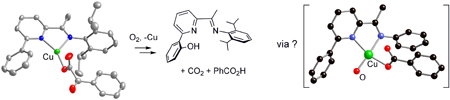
Figure. The
X-ray structure of a CuI-a-ketocarboxylate
complex, its reaction with O2 resulting in hydroxylation of
the appended arene ring, and the calculated structure of a [CuII-O-· « CuIII=O2-]+ intermediate
for one of two proposed reaction pathways.
These results have appeared online: "Copper(I)-a-Ketocarboxylate
Complexes: Characterization and O2 Reactions That Yield Copper-Oxygen
Intermediates Capable of Hydroxylating Arenes" Hong, S.; Huber, S. M.; Gagliardi,
L.; Cramer, C. C.; Tolman, W. B. J. Am. Chem. Soc. 2007, 129,
DOI: 10.1021/ja0760426.
|