11/28/2007
Storable Sources of Atomic Fe-,
Nb-, Ta- and other Wonders of Transition Metal Chemistry
Recent research from the group of Professor John E Ellis.
In recent years, we have prepared the first examples
of homoleptic polycyclic aromatic hydrocarbon, or polyarene, complexed
metal anions, in which the polyarene ligands often undergo facile and complete
displacement by good acceptor ligands, such as CO, PF3, P(OR)3,
CNR, olefins and potentially many others. Thus, these unprecedented polyarenemetalates
function as previously almost inaccessible transition metal atomic anions for
use in conventional inorganic/organometallic syntheses. Compounds of this
type are now known only for the elements, Ti, Zr, Hf, V, Nb, Ta, Fe and
Co, all of which have been originally prepared in this laboratory. So there
is a considerable amount of exciting exploratory research remaining to
be carried out in this relatively new area of chemistry!
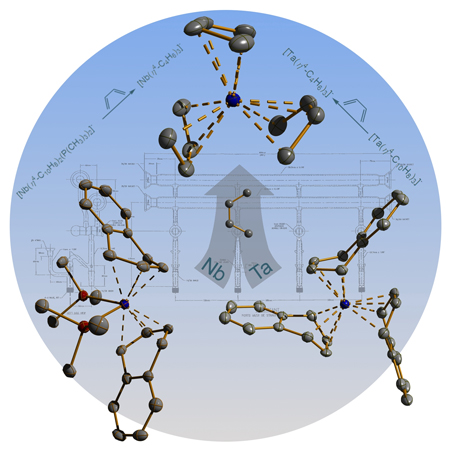
Very recently, graduate students William Brennessel,
Robert Jilek, and Victor Sussman, working in the laboratory of Professor
John Ellis, prepared dark red bis(anthracene)ferrate(1-), 1,
the first homoleptic polyarene complex of iron and bright orange bis(naphthalene)bis(trimethylphosphane)niobate(1-), 2,
an unprecedented niobium naphthalene complex, respectively, and examined
their reactions with 1,3-butadiene, an important hydrocarbon in industry,
organic synthesis, and organometallic chemistry. In these experiments the
butadiene rapidly displaced all ligands from highly air-sensitive 1 and 2 to
afford good yields of the first isolable and structurally characterized
anionic homoleptic butadiene metal complexes: the 17-electron pink-red
[Fe(C4H6)2]-, 3, and
the 18-electron colorless [Nb(C4H6)3]-,4.
Thus, in these reactions 1 and 2 function
as sources of atomic Fe- and Nb-, respectively. Amazingly, 3, 4,
and the analogous tantalum complex prepared in this study, [Ta(C4H6)3]-,
are precedented by only two other well-defined homoleptic 1,3-butadiene
transition metal complexes, the 18-electron neutral species, M(C4H6)3,
M = Mo, W. Syntheses of the latter compounds represented a significant
early triumph of the now classic metal vapor synthesis (MVS) method, which
has greatly contributed to our fundamental understanding of inorganic and
organometallic chemistry through the discovery of new classes of "textbook"
molecules. Interestingly, prior attempts to obtain homoleptic butadiene
complexes of Fe, Nb or Ta by the MVS route failed, so in this regard
the syntheses of 3, 4, and [Ta(C4H6)3]- represent
a significant new advance in our polyarenemetalate research, which
is generously supported by the National Science Foundation.
Also see the “Research News” report of
27 March 2007 describing recent results from the laboratory of
Professor Doreen G. Leopold on an amazing benzeneniobate(1-), another
formally Nb(I-) complex, which may be regarded as an extraordinarily
electron deficient relative of 2.
A graphic depicting the syntheses and structures of
the niobium and tantalum complexes is shown below. This artwork was prepared
by Dr. Victor Sussman, who now is a research scientist at the Dow Chemical
Company.
The iron chemistry has recently been published: “Bis(1,2,3,4-h4-anthracene)ferrate(1-): A Paramagnetic Homoleptic Polyarene Transition-Metal Anion,” Brennessel, W. W.; Jilek, R. E.; Ellis, J. E. Angew. Chem. Int. Ed. 2007, 46, 6132-6136 (http://dx.doi.org:10.1002/anie.200701353). The niobium and tantalum chemistry will soon appear online: “From Storable Sources of Atomic Nb- and Ta- to Isolable Anionic Tris(1,3-butadiene)metal Complexes: [M(h4-C4H6)3]-, M = Nb, Ta,” Sussman, V. J.; Ellis, J. E. Angew. Chem. Int. Ed. (http://dx.doi.org:10.1002/anie.200703887). This article was selected as a “Very Important Paper,” a status achieved by less than 5% of the communications published in Angewandte Chemie.
|