10/3/2007
FeV=O in
Bio-inspired Olefin Epoxidation Catalysis.
Recent Research from the group of Professor Lawrence
Que.
Nearly quantitative conversion of cyclooctene to
its epoxide (200 turnovers) can be obtained within one minute at 0 oC
using H2O2 as oxidant and [FeII(TPA)(NCMe)2]2+
(for structure of TPA, see scheme) as catalyst (0.5 mol% in 1:2 CH3CN/HOAc
solvent). These results by postdoctoral associate Ruben Mas-Balleste will soon
appear in the Journal of the American Chemical Society as part of an effort to discover efficient methods
for catalytic epoxidation using inexpensive oxidants. The approach in Prof.
Larry Que’s laboratory is inspired by iron enzymes that activate O2.
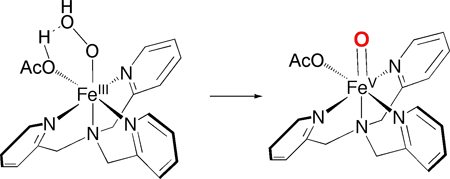
Insight into the nature of the active species has been gleaned from
spectroscopic experiments at low temperature. In the absence of substrate, FeIII-OOH
and FeIV=O intermediates can be observed, but neither is kinetically
competent as the active epoxidizing agent. Several lines of evidence support
the hypothesis that HOAc binds to the FeIII-OOH moiety and promotes
by protonation the heterolysis of the O-O bond to form an O=FeV-OAc
oxidant. Such a proton-assisted O-O bond cleavage is generally accepted to be
the key step in generating the active oxidant in the cytochrome P450 mechanism.
|