10/22/2007
Playing
Rubik's Cube with Nanoparticles
Recent Research from the group of Professor Andreas
Stein.
Atomic and ionic
crystals pack atoms in a wide range of periodic motifs. When the building
blocks are larger than atoms – uniform, spherical nanoparticles or
colloids with sizes between around 10 and 100 nanometers – the resulting
colloidal crystals typically assume a dense face-centered-cubic (fcc) order. Such colloidal crystals are of great interest
in the growing field of photonics, where they may be used to manipulate
the propagation or emission of light. However, if the choice of a photonic
crystal structure is limited to fcc, optical properties are also restricted.
Graduate student
Fan Li and senior Sarah Delo in Professor Stein's group recently discovered
an unusual approach to assemble nearly cubic nanoparticles into ordered
arrays with simple cubic rather than fcc symmetry. Surprisingly, they achieved this with a
template or mold consisting of fcc-packed
polymer spheres. After these were infiltrated with a surfactant and with
precursors for titania-phosphorus oxide composites and subsequently heated
to 400 °C, the majority of the product showed periodic arrays of tiny cubes
as depicted in the scanning electron micrograph in Figure 1. Multiple layers
are clearly visible, in which square arrangements of particles sit directly
on similarly arranged cubes in adjacent layers, forming a simple cubic
structure.
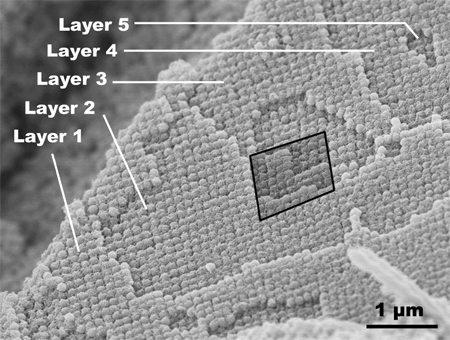
Figure 1. Titania-phosphorus oxide cubes in simple cubic pattern.
On
the basis of structural and compositional analyses, Stein and coworkers
proposed the mechanism outlined in Figure 2 for the product formation.
When the precursor hardens within the fcc colloidal
crystal template it initially forms a three-dimensionally ordered macroporous
(3DOM) skeleton. During sample heating the skeletal walls shrink and
eventually break down into small, nearly identical building blocks,
still in an fcc arrangement.
However, at the processing temperature, the phosphorus oxide component
forms a liquid phase. Resulting capillary forces move the particles
closer together, so that adjacent layers merge, forming the simple cubic
array.
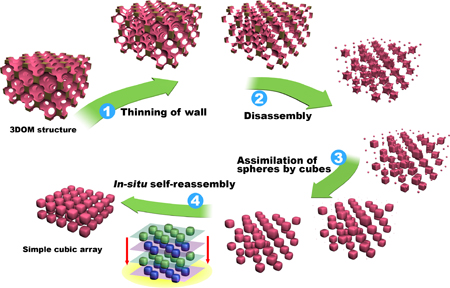
Figure 2. Proposed mechanism for the structural transformation from face-centered cubic to simple cubic.
In their publication (Angew. Chem. Int. Ed. 2007, 46, 6666, http://dx.doi.org/doi:10.1002/anie.200701553),
Li et al. conclude that "Even though the structures created in
the present work are far away from the perfection necessary for
photonic crystals, an adaptation of the self-reassembly method
may provide a faster, low cost alternative to produce simple cubic
photonic crystals that are normally prepared by elaborate micromachining,
layer-by-layer lithography, holography and macroporous silicon
etching, all expensive and time-consuming methods." This
work was also highlighted in a Nature New & Views article (Nature
2007, 449, 550. http://dx.doi.org/doi:10.1038/449550a)
|