09/25/2007
Nanocrystal Growth by Oriented Aggregation
Recent Research from the group of Professor
R. Lee Penn.
Oriented aggregation is an important, nonclassical crystal growth mechanism
by which nanocrystals grow, defects are formed, and unique - often symmetry-defying
- crystal morphologies can be produced. This growth mechanism involves the
irreversible and crystallographically specific self-assembly of primary nanocrystals
and results in the formation of new single crystals, twins, and intergrowths.
It offers a route by which nanocrystal size, morphology, and microstructure
can be controlled. Recently, the Penn group demonstrated particle size control
by exploiting oriented aggregation in the formation of goethite (alpha-FeOOH)
nanorods from ferrihydrite nanoparticles. Specifically, they showed that
the size of the goethite nanocrystals formed depends directly on the size
of the precursor ferrihydrite nanoparticles (figure 1). Growth by oriented
aggregation is consistent with second-order kinetics with respect to the
concentration of the primary nanoparticles, and the Penn group has further
shown that the overall rate constant for growth by oriented aggregation is
strongly size dependent (figure 2). These results explain the common observation
that early nanocrystal growth in solvothermal conditions is dominated by
oriented aggregation and that its contribution to overall growth slows as
a function of continued crystal growth.
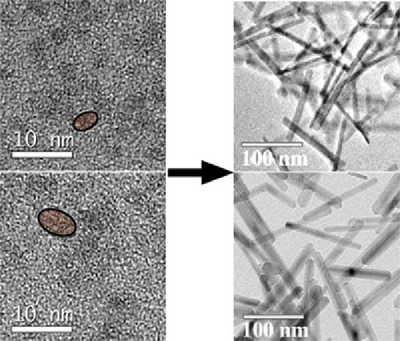
Figure 1: Transmission electron micrographs of goethite nanorods (right)
produced from ferrihydrite nanodots (left). Smaller nanorods are produced
by oriented aggregation of the smaller nanodots (upper), and larger nanorods
are produced by oriented aggregation of the larger nanodots (lower). Ref.
Penn et al., 2006.
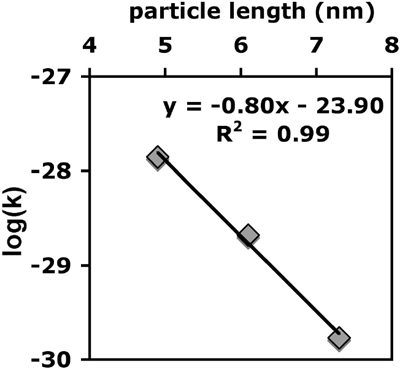
Figure 2: Plot of the log of the rate constant for oriented aggregation
(log(k)) versus average particle length demonstrating the strong size dependence
of the kinetics of growth by oriented aggregation. The linear relationship
between log(k) and size is consistent with DLVO predictions. Ref. Penn et
al., IN PRESS.
References:
Size Dependent Kinetics of Oriented Aggregation. R. L. Penn, K. Tanaka,
and J. J. Erbs (IN PRESS) Journal of Crystal Growth.
Controlled growth of alpha-FeOOH nanorods by exploiting oriented aggregation.
R. Lee Penn, J. Erbs, and D. Gulliver, (2006). Journal of Cyrstal Growth, 293, 1-4.
Undergrad authors are shown in BOLD.
|