08/20/2008
Cell Penetrating Peptides
Recent
research from the groups of Professors Mark
Distefano and George
Barany done in collaboration with Professor Elizabeth Wattenberg.
Prenylation
is a common post-translational protein modification in eukaryotic cells.
When prenylation occurs an enzyme adds a 15 or 20 carbon chain near the C-terminus
of a protein. The modification controls the protein’s localization inside
of the cell and is involved in directing other cellular events including
some that are involved in the regulation of cancer proliferation.
Currently
researchers in the Distefano lab have discovered other interesting properties
of prenylated molecules. Their research has uncovered a series of prenylated
peptides that have cell penetrating properties. These peptides could open
the door for further study on the properties of larger prenylated proteins
in cells, including these proteins’ participation in cellular signaling
pathways involved in cancer proliferation. This information could lead
to new cancer therapies and strategies for cancer prevention.
Cell
penetrating peptides were prepared by Dan Mullen of Professor Barany’s
group and James Wollack of the Distefano group. The cell penetrating
properties of these peptides were investigated in Professor Wattenberg’s
group by her student Nicholette Zeliadt in collaboration with James Wollack.
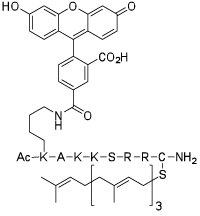 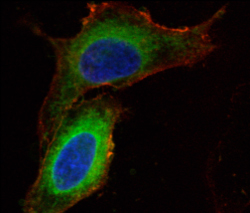
Right: HeLa
cells treated with the pictured peptide. Green areas are the peptide localized
between the nucleus and the plasma membrane. The plasma membrane is stained
red and the nucleus is stained blue.
Left:A fluorescently labeled cell penetrating prenylated peptide
|