07/10/2008
Methyl Anion
Dethroned as the Most Basic Species
Recent research from the group of Professor Steven Kass.
After decades as the champion of proton acceptors, the methyl anion is finally
playing second fiddle to the gas-phase lithium monoxide anion. In a joint
effort between University of Minnesota researchers Zhixin Tian and Prof.
Steven R. Kass and collaborators at the University of Sydney,
the LiO- species was prepared and its acid-base properties were
measured to verify calculations that predicted its chemical prowess. Their
work was reported in The Proceedings of the National Academy of Sciences
(Proc. Natl. Acad.
Sci. USA 2008, 105,
7647) and highlighted this month in Chemical and Engineering
News (C&EN June 9, 2008).
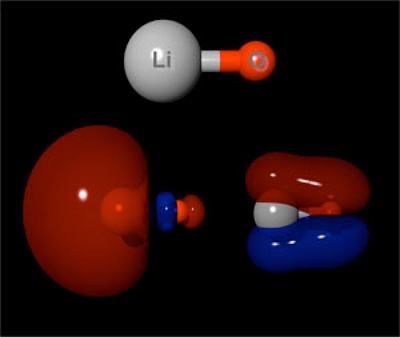
LiO- ANION (top),
a biradical species, has two highest occupied molecular orbitals (bottom,
left and right) containing one unpaired electron each.
|