11/05/2008
One monomer, two polymers
Recent research from the group of Professor Marc Hillmyer.
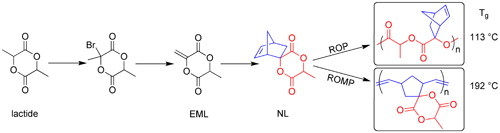
Lactide is the cyclic dimer of lactic acid, a biorenewable hydroxy acid. The ring-opening polymerization (ROP) of lactide is carried out commercially to produce polylactide (PLA), a biodegradable polyester. PLA is also a sustainable material due to its non-petrochemical origins and is being implemented in applications ranging from clothing to packaging to compostable trash bags. In an effort to modify and expand the properties of PLA, Dr. Feng Jing, a postdoctoral research associate in Professor Marc Hillmyer's research group, recently showed that lactide could be dehydrogenated to give exomethylene lactide (EML) through a bromination/dehydrobromination sequence. He went on to show that EML reacts with dicyclopentadiene in a Diels-Alder reaction to give the new bifunctional monomer norbornene lactide (NL). ROP through the cyclic ester functionality in NL gives a substituted polylactide with a glass transition temperature (Tg) or softening point of 113 °C, more than 50 °C higher than PLA. This new material could be useful applications that require higher thermal stability than that offered by PLA. Opening of the norbornene ring in NL by ring-opening metathesis polymerization (ROMP) yields a substituted polyalkeneamer with a remarkably high Tg of 192 °C. Copolymerization of NL with cyclooctene by ROMP gives a lactide functionalized rubbery material that can be used as a toughening agent for PLA. Composites produced by the polymerization of lactide in the presence of this reactive copolymer are significantly tougher than the parent PLA at relatively low levels rubber incorporation. A complete description of this work was recently communicated to the Journal of the American Chemical Society:
Jing, F.; Hillmyer, M. A. – A Bifunctional Monomer Derived Directly from Lactide for Toughening Polylactide – J. Am. Chem. Soc. 2008, 130, 13826–13827.
|