04/14/2009
Breaking Strong C-H and O-H Bonds with a Diiron(IV) Complex
Recent research from the research group of Professor Larry
Que.
In a paper just published in Nature Chemistry (DOI:
10.1038/nchem.162), graduate student Dong
Wang in Professor Larry Que’s group reported the electrochemical generation
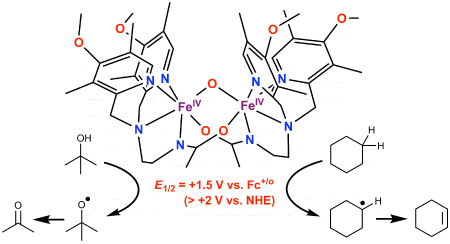
of a (μ-oxo)diiron(IV)
complex with a redox potential of +1.50 V vs. ferrocene (> +2 V vs. NHE),
making this the complex with the highest FeIV/III potential experimentally
determined to date. This project was inspired by the chemistry of the enzyme
methane monooxygenase, which converts methane to methanol via a diiron(IV)
intermediate called Q. Low-temperature bulk electrolysis of the diiron(III)
precursor at very positive applied potentials afforded its two-electron oxidized
diiron(IV) form in high yield. This species has been characterized by Mössbauer
spectroscopy (by Dr. Audria Stubna and Prof. Eckard Münck of Carnegie Mellon
University) and EXAFS analysis (by graduate student Erik Farquhar). Driven
by its high redox potential, this species oxidizes cyclohexane and its perdeuterated
analog (bond dissociation energies of 99.3 and 100.2 kcal/mol, respectively)
to give dehydrogenated products at reaction rates up to 1,000-fold higher
than those of previously reported FeIV=O complexes. More surprisingly,
this highly reactive complex attacks the strong O-H bonds in tert-butanol
(rather than its weaker and more numerous C-H bonds), representing for the
first time a new type of reactivity for iron complexes. Illustrated below
are the structure of this diiron(IV) complex and its novel reactivity.
|