|
08/07/2009
Stringing up nanobeads
Recent research from the research group of Professor Andreas Stein.
Spherical colloids with diameters of a few hundred
nanometers (several hundred times smaller than the thickness of a human hair)
are useful building blocks for self-assembly into functional structures.
They can be likened to atoms but on a larger scale. Just like metal atoms
that assemble into close-packed structures, they can also pack into regular
patterns. Opals are natural examples of close packed colloidal spheres, and
their brilliant colors ("opalescence") arise from the interaction
of light with the periodic sphere structures. Many examples of close-packed
colloids can be found in the literature, but more open structures or analogs
of molecules, in which "colloidal atoms" are connected with specific
directionality are more difficult to obtain.
In a recent paper published in the Journal
of the American Chemical Society 2009, 131, 9920, graduate
student Fan Li and Professor Andreas Stein describe a novel approach to
connect colloids into conjugated clusters and chains of various geometries,
using a process called
"capillary condensation" to provide the glue and to impart clusters
with specific directional interactions. Capillary condensation involves selective
condensation of a vapor below its saturation vapor pressure in cavities or
between surfaces. Dichloromethylvinylsilane vapor
was deposited between spin-coated aggregates of polymer spheres. The vapor
condensed primarily between sphere contacts and reacted to form a thin coating
in these regions that linked the spheres together and stabilized the clusters.
This process occurred even if spheres were slightly separated from each other.
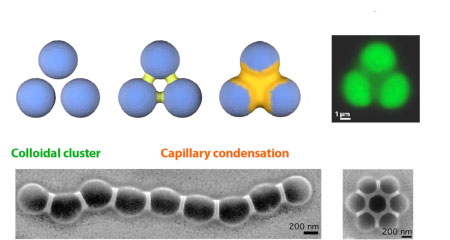
The figure below shows a schematic drawing of
a colloidal cluster with three spheres that are linked by the capillary condensation
process. When fluorescent polymer spheres are connected, the "nanoglue" shows
up as dark regions in the fluorescence image. In the scanning electron images
of a linked chain and a seven-particle cluster, the glue appears as lighter
regions. Either the coated or the non-coated regions can then be selectively
functionalized to provide contact points for further reactions, for example,
if clusters are intended to be linked up into more extended structures via
directional interactions. This method provides a new tool toward
mimicking molecular assembly at the colloidal scale and toward the realization
of rational colloid assembly processes by true designer pathways.
|