|
12/18/2009
Mechanism of Water Oxidation for the Ru-Hbpp Catalyst
Recent research from the research groups of Professors Laura
Gagliardi and Christopher
J. Cramer.
The binuclear
catalyst in,in-{[RuII(trpy)(H2O)]2(μ-bpp)}3+ (Ru-Hbpp;
trpy is 2,2':6',2"-terpyridine, bpp is 2,6-bis(pyridyl)-pyrazolate) facilitates
the generation of molecular oxygen from water. This water oxidation step
is the key bottleneck currently associated with the development of commercial
light harvesting devices for the photo-production of H2 from water.
Through a combination of density functional theory and multireference wave
function theory computations carried out by Mehmed Z. Ertem and Tanya K.
Todorova, the Gagliardi and Cramer groups were able to rationalize mechanistic
and kinetic details consistent with experimental data developed in the group
of collaborator Antoni Llobet of the Institute of Chemical Research of Catalonia,
establishing an intramolecular pathway for O–O bond formation. This
work is described in a full paper recently published in the Journal of the
American Chemical Society, 2009, 131, 15176 (DOI: 10.1021/ja9036127)
and should prove particularly useful for the design of improved water-splitting
catalysts for future applications.
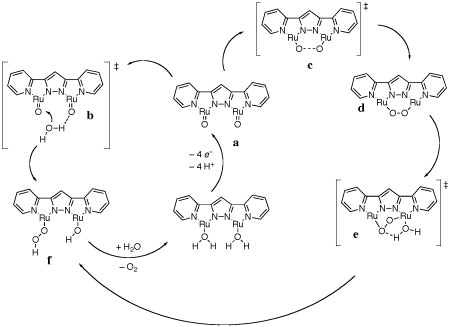
Theory indicates the active catalytic pathway
to be the clockwise (intramolecular) alternative above.
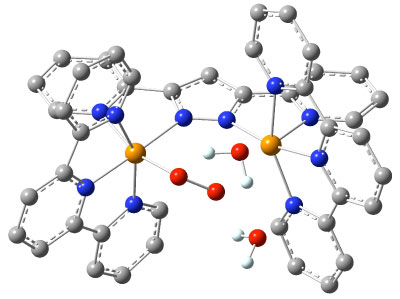
Rate-determining transition-state structure e for
oxygen generation from water by the binuclear Ru-Hbpp catalyst (ligand H
atoms omittted for clarity).
|