02/17/2009
Reduction of nitrous oxide to dinitrogen by
a mixed valent tricopper-disulfido cluster
Recent research from the groups of Professor Christopher
Cramer and Professor William
Tolman.
The greenhouse gas N2O is converted
to N2 under mild conditions by a μ-sulfido-tetracopper active site in the enzyme
nitrous oxide reductase (N2OR) via a process postulated to involve μ-1,3
coordination of N2O to two Cu(I) ions. In efforts to develop synthetic
models of the site with which to test mechanistic hypotheses, Dr. Itsik Bar-Nahum
and Aalo Gupta in Prof. Tolman’s group have prepared and fully characterized
a localized mixed valent Cu(II)Cu(I)2 cluster bridged in μ-η2:η1:η1 fashion
by disulfide, [L3Cu3(μ-S2)]X2 (L
= 1,4,7-trimethyl-triazacyclononane, X = O3SCF3- or
SbF6-). This cluster exhibits spectroscopic features
similar to those of the active site in N2OR and reacts with N2O
to yield N2 in a reaction that models the function of the enzyme.
Computations by Dr. Stefan Huber and Mehmed Z. Ertem in Prof. Cramer’s group
implicate a transition state structure that features μ-1,1-bridging
of N2O via its O-atom to a [L2Cu2(μ-S2)]+ fragment and
provide chemical precedence for an alternative pathway for N2O
reduction by N2OR. This work is described in a communication just
published online in the Journal of the American Chemical Society (DOI: 10.1021/ja808917k).
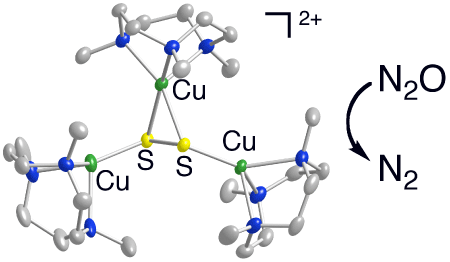
Structure of the new cluster that reduces N2O to N2.
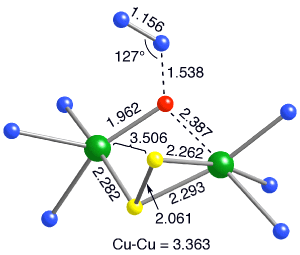
Calculated structure of the transition state
for N2O reduction by a dinuclear fragment of the tricopper cluster
(ligand C atoms omittted for clarity).
|