02/10/2009
Shape-Selective C–H Oxidation
Recent research from the group of Professor Lawrence Que.
In a VIP paper just published in Angewandte Chemie (DOI: 10.1002/anie.200805342), graduate student Anusree
Mukherjee in Larry Que’s group and coworkers describe the first example
of shape-selective oxidation of an aliphatic hydrocarbon by an O2-activating
iron(II) complex. In Nature, many iron enzymes tap the oxidizing potential
of O2 to perform selective substrate oxidations. Oxygenases
that require α-keto
acid as a cofactor comprise a subset of these enzymes. We have synthesized
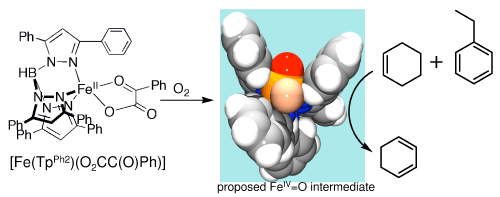
the complex, [Fe(TpPh2)(O2CC(O)Ph)] (TpPh2 =
tris(3,5-diphenylpyrazolyl)borate; see scheme below), to serve as a model
for the active site of such enzymes. Indeed we have shown this complex
to be able to activate O2 to form a transient FeIV=O
oxidant that effects intramolecular hydroxylation of a ligand phenyl ring.[1]
Recently,
we have observed that this complex is also able to carry out intermolecular
oxidations of added hydrocarbon substrates. In other words, the added substrate
can intercept the oxidant generated by O2 activation and be
itself oxidized instead of the ligand phenyl ring. Indeed, C-H bonds as
strong as those of cyclooctane (96 kcal/mol) are attacked. Most strikingly,
when cyclohexene and ethylbenzene are used as substrates in a competitive
oxidation experiment, only cyclohexene is oxidized despite the similar
strengths of their weakest C-H bonds. We postulate this selectivity to
be based on shape, the shape of a cleft formed by two phenyl groups of
the TpPh2 ligand that flank the putative FeIV=O oxidant,
which favors oblate spheroidal substrates.
Shape
selectivity has been very difficult to accomplish in biomimetic chemistry,
particularly without a significant effort in synthesis to assemble the
target molecule. In our case, [Fe(TpPh2)(O2CC(O)Ph)]
is easily synthesized from its components by a one-step procedure. What
is amazing is that so simple a complex can achieve shape-selective discrimination
of substrates, similar to that often associated with more complex enzyme
active sites.
[1] M. P. Mehn, K. Fujisawa, E. L. Hegg, L. Que, Jr., J. Am. Chem. Soc. 2003, 125,
7828.
|