01/15/2009
Bio-inspired iron-catalyzed cis-dihydroxylation of an arene
Recent research from the group of Professor Lawrence Que.
In a paper just published in Chemical Communications (Chem. Commun. 2009,
50-52) and designated as a HOT article by the RCS journal,
graduate student Yan Feng and coworkers reported the first example of a synthetic
iron complex that activates H2O2 to effect the cis-dihydroxylation
of naphthalene. In Nature, the bio-degradation of aromatic compounds is initiated
by Rieske dioxygenases, which catalyze the cis-dihydroxylation of
an arene double bond. As found for naphthalene 1,2-dioxygenase (NDO), the
best structurally characterized of this class, these enzymes have a mononuclear
non-heme iron active site where O2 activation occurs. To mimic
enzyme function, the complex [FeII(TPA)(NCMe)2](OTf)2 (1)
was used as catalyst and H2O2 as oxidant to convert
naphthalene to its cis-1,2-dihydrodiol derivative, demonstrating this
transformation for the first time by an iron complex (Figure 1). 18O
labeling studies showed that one oxygen atom in the cis-diol product
derived from H2O2 and the other from H2O.
This unique labeling pattern required the postulation of an cis-HO-Fe(V)=O
oxidant that is formed from the water-assisted cleavage of an Fe(III)-OOH
intermediate (Figure 2). The proposed oxidant is analogous to the OsO4 reagent
that is well established to carry out the cis-dihydroxylation of olefins
(but not arenes). This research effort is supported by the US Department
of Energy and aimed at developing oxidation catalysts with economical and
environmentally friendly metal centers.
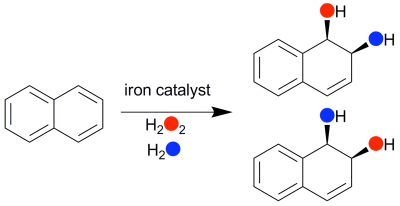
Figure 1. Naphthalene cis-dihydroxylation
catalyzed by 1
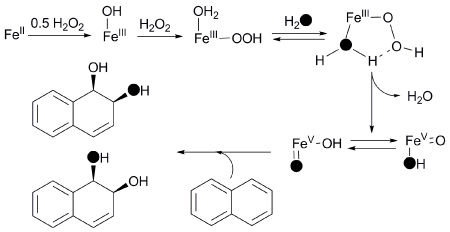
Figure 2. Proposed mechanism for
naphthalene cis-dihydroxylation by 1.
|