06/16/2009
Making Modern Materials
by the Metathesis Manipulation of a Monoterpene
Recent research from the research groups of Professors Marc Hillmyer and Thomas Hoye.
Shingo Kobayashi and Cheng
Lu working in the labs of Professors Marc Hillmyer and Tom Hoye recently
demonstrated the controlled polymerization of a cyclic diene prepared from
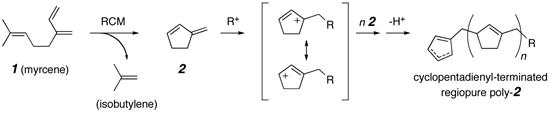
the ring-closing metathesis (RCM) reaction of myrcene, a naturally occurring
hydrocarbon produced by a variety of plants or by the rearrangement of
pinene, the principal component of turpentine. The cyclic diene 3-methylenecyclopentene
(2) was synthesized from myrcene (1) by means of RCM chemistry.
The monomer 2 could be polymerized by chain polymerization techniques
using radical, anionic, and cationic initiators. The polymerization of 2 proceeded
in "living" fashion in both anionic and cationic polymerization systems
and afforded polymers in quantitative yield with narrow molecular weight
distributions and predictable molecular weights. Furthermore, the living
cationic polymerization of 2 proceeded smoothly to afford regiopure
polymers having thermal properties consistent with a stereoregular structure.
The second product in this RCM reaction is isobutylene, a monomer that
can be converted into butyl rubber. In principle, this chemistry will allow
us the overall transformation of the starting material 1 into polymeric
materials with perfect atom economy.
Basic research that underpins
the development of new materials from renewable resources is the focus of
the new Center for Sustainable Polymers at the University of
Minnesota.
This work was recently reported in the Journal of the American Chemical
Society (J. Am. Chem. Soc. 2009, 131, 7960–7961).
|