05/29/2009
Crystal structure of octabromoditechnetate(III)
and a multi-configurational quantum chemical study of the quadruply bonded
[M2X8]2- dimers (M = Tc, Re; X = Cl, Br)
Recent research from the research group of Professor Laura Gagliardi.
Understanding the properties of compounds that contain metal-metal bonds is fundamental to interpreting structural and bonding properties, catalysis, metal surface chemistry, and magnetism. The discovery of dirhenium(III) complexes with metal-metal quadruple bonds foreshadowed a revolution in the study of metal-metal bonds and the identification of novel types of bonding. The importance of quantum chemistry to this area of science is not only based on its ability to solve the quantum-mechanical equations to a good degree of approximation for complex molecules, but also on the fact that the field can now perform theoretical simulations of real benefit to the experimental community. Technetium chemistry represents a challenge for experimentalists and the interplay between theory and experiment is of extreme importance in such a case.
The single-crystal X-ray
structure of carmine-red [Tc2Br8]2− has
been determined almost three decades after the original report of its
synthesis. Frederic Poineau, Paul M. Forster, Alfred P. Sattelberger
and Kenneth R. Czerwinski at the Department of Chemistry, University
of Nevada Las Vegas, have prepared the technetium(III) compound (n-Bu4N)2[Tc2Br8]
by metathesis of (n-Bu4N)2[Tc2Cl8]
with concentrated aqueous HBr in acetone and recrystallized from acetone-diethyl
ether solution. The structure of [Tc2Br8]2− was
previously determined via EXAFS methods on an acetone free sample
of (n-Bu4N)2[Tc2Br8] and
the two methods give essentially identical results for the quadruply bonded
anion.
Laura Gagliardi, at the Department of Chemistry,
University of Minnesota, has performed multiconfigurational quantum chemical
calculations of the molecular structure and electronic spectra of the [Tc2Br8]2− compound
and comparative calculations on the [Re2Cl8]2− [Tc2Cl8]2− [Re2Br8]2− compounds.
Novel
Tc-Tc compounds are now under investigation.
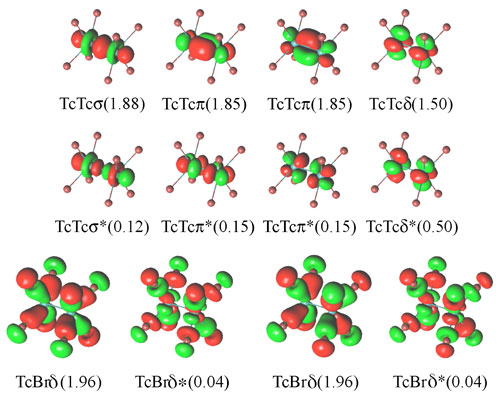
Figure.
Active orbitals for [Tc2Br8]2-
with their occupation numbers in the ground state.
Their results have just appeared in Dalton Transactions
among 'The hottest science', as recommended by the referees, published in Dalton
Transactions. DOI: 10.1039/b902106j
|