05/22/2009
More Consistent van der Waals Radii
Recent research from the research groups of Professors Donald
Truhlar and Christopher
Cramer.
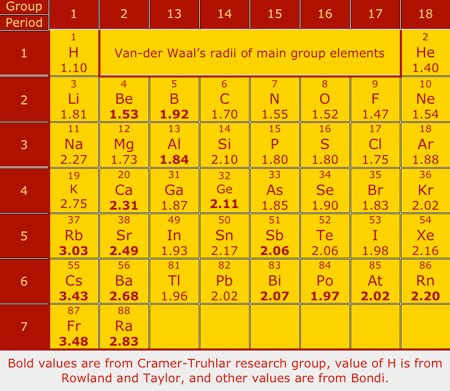
Atoms aren't actually uniform spheres, but treating them as such with a measure known as the van der Waals radius is crucial for molecular modeling. Although chemists have calculated numerous values for van der Waals radii of the elements using different approaches over the years, no consistent set exists across the periodic table. Donald G. Truhlar and colleagues of the University of Minnesota, Twin Cities, have now developed a theoretical method that generates van der Waals radii for all the main-group elements (J. Phys. Chem. A, DOI: 10.1021/jp8111556). For many elements, van der Waals radii are calculated by measuring the spaces between nonbonded atoms in crystals or the gas-phase equilibrium distances between nonbonded atoms in van der Waals complexes. But different methods produce different values. The most widely used collection of radii was calculated in the early 1960s by Shell chemist Arnold Bondi on the basis of crystallographic data (J. Phys. Chem. 1964, 68, 441). However, Bondi's data only included radii for 28 of the 44 main-group elements. Truhlar's method produces van der Waals radii that agree with Bondi's data and also generates values for the 16 remaining main-group elements. Filling in the "radius gaps" should be "useful to researchers in myriad research areas," Truhlar says.
|