|
09/06/2009
A human diiron enzyme that controls cell growth
Recent research from the research group of Professor
Lawrence Que.
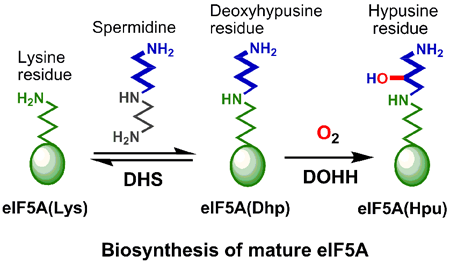
Deoxyhypusine hydroxylase (DOHH) is an iron-requiring
enzyme that catalyzes the hydroxylation of a deoxyhypusine residue in the
final step of the maturation of eukaryotic translation initiation factor
5A (eIF5A). This transformation plays an essential role in the regulation
of eukaryotic cell proliferation, making DOHH an attractive target for anti-tumor
and anti-HIV therapies. However, little is known of the details of its iron
active site and reaction mechanism.
In a paper that has just appeared in Proceedings
of the National Academy of Sciences USA (PNAS 2009, 106,14814-14819;
doi:10.1073/pnas.0904553106), graduate student Van Vu and collaborators
at Carnegie Mellon University and the National Institutes of Health report
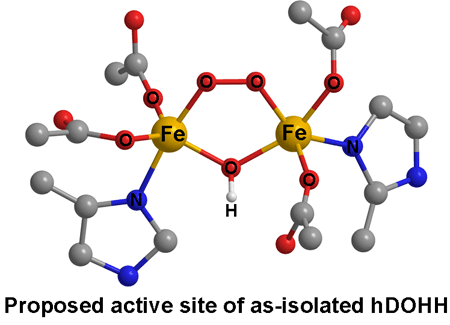
studies on the recombinant human enzyme (hDOHH) and find that hDOHH has
a non-heme diiron cluster in its active site. While similar active sites
have been found in other O2-activating non-heme diiron enzymes,
including methane monooxygenases from bacteria and fatty acid desaturases
from plants, hDOHH represents the first human hydroxylase demonstrated
to have such an active site. Thus the diiron motif is a recurring strategy
Nature employs to activate O2 in order to attack strong C-H
bonds, even in mammals.
Surprisingly, as isolated hDOHH has an unusual
blue color that persists at room temperature for several days. Spectroscopic
studies show that this chromophore arises from an unusually stable diiron-O2 adduct.
For comparison, O2 adducts of related diiron enzymes are more
reactive and can have lifetimes as short as milliseconds. Nevertheless, as
isolated hDOHH can in fact carry out the hydroxylation of the deoxyhypusine
residue in eIF5A. Future efforts are aimed at understanding why this peroxo
species is stable and how it becomes activated, thereby providing further
insight into the oxygen activation mechanisms of the non-heme diiron enzyme
family.
|