|
01/14/2010
Voltammetry at the Ultimate Limit of Low Polarity
Recent research from the research group of Professor Phil Buhlmann.
In work to be published shortly in the Journal of Electroanalytical Chemistry (doi:10.1016/j.jelechem.2009.12.006),
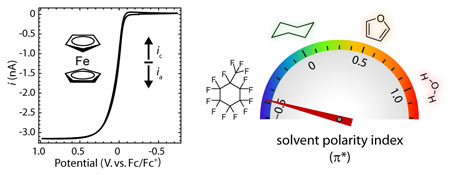
graduate student Eric Olson and co-workers describe the first cyclic voltammetry
in a fluorous solvent. Electrochemistry in organic solvents of low polarity
such as alkanes has been widely studied because of the unique solubility
and reactivity of analytes in these solvents. However, while many organic
solvents do not have a permanent dipole, the carbon–hydrogen bond
is readily polarizable. The extreme electronegativity of fluorine imparts
C–F bonds with very low polarizabilities, minimizing van der Waals
interactions and making perfluorocarbon solvents the least polar/polarizable
condensed phases known. This is exemplified by the π* star scale of solvent polarity/polarizability,
on which water takes the value of 1.08, cyclohexane defines 0, and perfluorooctane
can be found at -0.41. Because of the low solubility of lipophilic electrolytes
in fluorous solvents, electrochemistry in these phases had not previously
been possible.
Using the novel fluorophilic electrolyte salt developed by Dr. Paul Boswell,
a recent graduate of the Buhlmann Group, cyclic voltammetry has been performed
with perfluoro(methylcyclohexane) as the solvent.
Surprisingly, the resulting voltammograms are easily modeled using existing
theory despite the unusual sample environment. Dr. Letitia Yao of the NMR
lab in the chemistry department performed DOSY 19F NMR spectroscopy
to verify that the addition of electrolyte does not significantly change
the solution viscosity. Moreover, dielectric spectroscopy was performed in
collaboration with Dr. Brad Givot from the 3M Corporate
Research Laboratory to confirm that addition of the fluorophilic electrolyte
does not increase the overall polarity of the fluorous solution.
The development of an electrolyte that permits electrochemistry in the fluorous
phase has not only advanced voltammetry to the ultimate limit of low polarity
in condensed phases, but it also opens doors for new sensors that utilize
fluorous materials as a sensing matrix. The Buhlmann group is currently performing
research to develop amperometric and voltammetric sensors that build upon
this work.
|