Main navigation | Main content
07/15/2010
Recent research from the research group of Professor Lawrence Que.
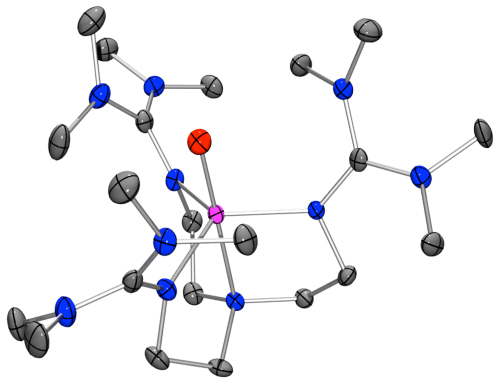
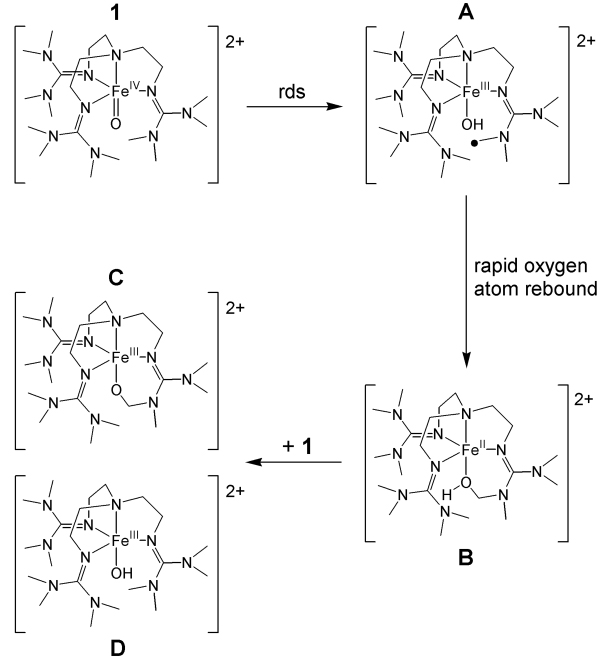
High-valent oxoiron(IV) species are considered to be the active oxidants in the catalytic cycles of a wide range of mononuclear non-heme oxygen activating enzymes. Several of these high-valent intermediates have now been spectroscopically characterized and found to have high-spin (S = 2) iron(IV) centers. In contrast, the overwhelming majority of synthetic oxoiron(IV) complexes exhibit an intermediate spin state (S = 1), with the only exceptions being the highly unstable [FeIV(O)(H2O)5]2+ and the trigonal bipyramidal complex [FeIV(O)(TMG3tren)]2+ (1). As a follow-up to their paper detailing the synthesis and spectroscopic characterization of the latter, Que group post-doc Dr Jason England together with collaborators at Carnegie Mellon University recently published an article in the Journal of the American Chemical Society (JACS, 2010, 132, 8635-8644, DOI: 10.1021/ja100366c) detailing the crystal structure of this complex, the first such example for a high-spin oxoiron(IV) complex. This was achieved as a consequence of a detailed analysis of the self-decay pathway of this complex, which indicated that the highly reactive oxoiron(IV) center reacted intramolecularly with the methyl substituents of the TMG3tren ligand. Retardation of this process by exploitation of the large kinetic isotope effect (24 at 25 °C) accompanying perdeuteration of the aforementioned methyl groups allowed crystals to be grown that were suitable for X-ray analysis. The solution of this first crystal structure of a high-spin (S = 2) oxoiron(IV) center represents a fundamental step on the path towards a full understanding of these pivotal biological intermediates.