Main navigation | Main content
Over the past decade, synthetic chemists have attempted to mimic the reactivity of the high-spin oxoiron(IV) intermediates found in many nonheme iron enzymes. However, functional model compounds of the nonheme halogenase enzymes, CytC3 and SyrB2, found to be responsible for the chlorination and bromination of important biological substrates, have proven elusive.
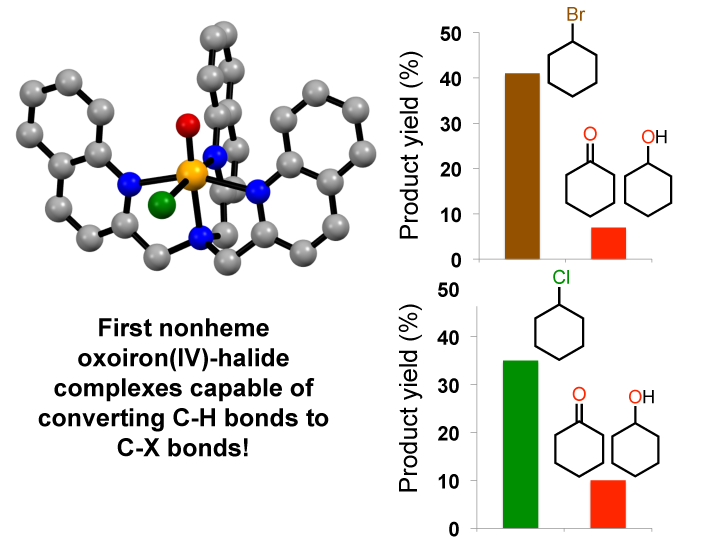
Now, for the first time, University of Minnesota graduate student Mayank Puri and post-doctoral associate Achintesh Biswas in the laboratory of Regents Professor Lawrence Que Jr. have characterized nonheme oxoiron(IV)-halide complexes that are both spectroscopic and functional models of the oxoiron(IV) intermediates observed in CytC3 and SyrB2. These reactive complexes are capable of cleaving the strong carbon-hydrogen bonds of cyclohexane and converting them into carbon-halogen bonds, with selectivity over the competitive formation of carbon-oxygen bonds.
This work was carried out in collaboration with Professor Yisong Guo and graduate student Ruixi Fan at Carnegie Mellon University. The findings have recently been published in the Journal of the American Chemical Society (DOI: 10.1021/jacs.5b11511). This report follows up on a previous study by the Que group involving the synthesis of the first high-spin nonheme oxoiron(IV) complex that models both the electronic structure and reactivity of the nonheme iron enzyme, taurine dioxygenase (J. Am. Chem. Soc. 2015, 137, 2428 – 2431).