Main navigation | Main content
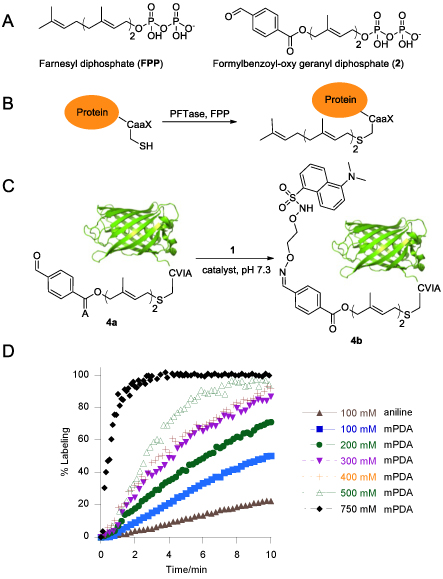
Imine-based reactions are useful for a wide range of bioconjugation applications. Although aniline is known to catalyze the oxime ligation reaction under physiological conditions, it suffers from slow reaction kinetics, specifically when a ketone is being used or when hydrazone-oxime exchange is performed. In a recent paper, published in Bioconjugate Chemistry (Bioconjugate Chem., 2013, 24 (3), pp 333-342, DOI: 10.1021/bc3004167), graduate students Mohammad Rashidian, Mohsen Mahmoodi and Jon Dozier, who are advised by Professor Mark Distefano, in collaboration with Professor Carston Wagner and Rachit Shah from the Department of Medicinal Chemistry, recently reported the discovery of a new catalyst that is up to 15 times more efficient than aniline.
That catalyst, m-phenylenediamine (mPDA), was initially used to analyze the kinetics of oxime ligation on aldehyde- and ketone-containing small molecules. While mPDA is only modestly more effective than aniline when used in equal concentrations (~ 2-fold), its much greater aqueous solubility relative to aniline allows it to be used at higher concentrations, resulting in significantly more efficient catalysis. Results with this new catalyst with various aldehyde- and ketone-functionalized proteins indicate that mPDA can be used to accelerate selective protein modification reactions by more than an order of magnitude. Rashidian et al. demonstrated that this new catalyst can be used to install fluorophores and PEG polymer chains into therapeutically important proteins. Overall, this new catalyst should have a significant impact on the field of bioconjugation, where oxime ligation and hydrazone-oxime exchange are commonly employed.
Figure:
Kinetic analysis of oxime ligation with an aldehyde-functionalized protein. A) Structures of FPP and aldehyde analogue 2. B) Schematic representation of a prenylation reaction with a protein containing a CaaX box at its C-terminus. C) Schematic representation of oxime ligation with an aldehyde-functionalized protein 4a using either aniline or mPDA as the catalyst. D) Kinetic analysis of oxime ligation between 4a (10 µM) and 1 (50 µM) using either aniline or different concentrations of mPDA.