Main navigation | Main content
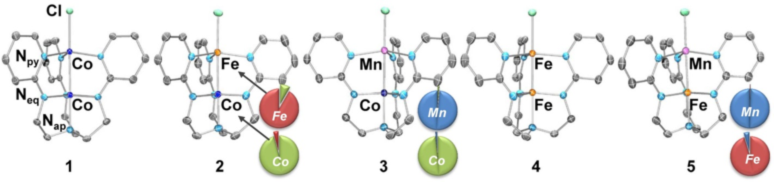
In a paper published in the Journal of the American Chemical Society, researchers report a five-membered family of homo- and heterobimetallic complexes using the first-row transition ions: Mn(II), Fe(II), and Co(II). The bimetallic complexes all showed a high degree of selectivity in the metal-binding sites, despite the fact that these metal ions have similar covalent radii and readily undergo ligand exchange reactions. This study established magnetic and bonding properties for specific metal-metal pairings, which holds promise for achieving predictable and precise control of cluster properties through metal atom substitution.
This work is a collaboration among the groups of Professor Connie Lu, Professor Laura Gagliardi, and Eckhard Bill, Ph.D. (Max Planck for Chemical Energy Conversion). Graduate student Steve Tereniak designed and executed the syntheses. Graduate student Becky Carlson performed the theoretical calculations with assistance from Rémi Maurice, Ph.D., and Hyun Jung Kim. The anomalous X-ray scattering experiments were performed by Laura Clouston (Lu group), Victor Young Jr., Ph.D., and Yu-Sheng Chen, Ph.D. (ChemMatCARS).
The paper can be viewed here.
Figure caption. Five-membered family of bimetallics featuring Mn(II), Fe(II), and Co(II). For the heterobimetallic complexes, the pie charts depict the percentages of each metal (Co in green, Fe in red, and Mn in blue) at each binding site as determined by X-ray anomalous dispersion studies.