Main navigation | Main content
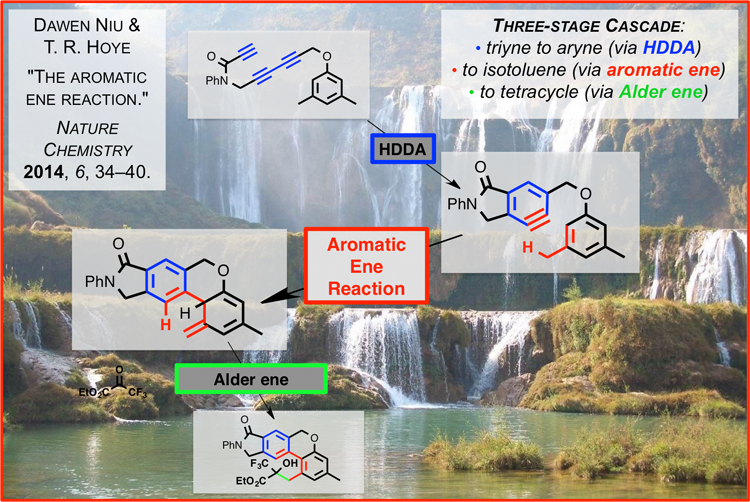
Dawen Niu, working in the laboratory of Professor Thomas Hoye, has discovered the first examples of the title reaction that proceed with high efficiency [Nature Chemistry, January 2014 (doi: 10.1038/nchem.1797)]. They have established that benzyne derivatives formed by the hexadehydro-Diels-Alder (HDDA) reaction [Nature (doi:10.1038/nature11518)] will engage a pendant arene ring in an "ene reaction" fashion. The resulting isotoluene intermediate can be further trapped in productive ways. This energetically favorable "ene-upon-ene cascade reaction involves the overall formation of four carbon–carbon bonds and three rings, requires no external reagents, and generates no by-products."1
1"The aromatic ene reaction," Niu, D.; Hoye, T. R. Nature Chem. 2014, 6, 34–40. http://www.nature.com/nchem/journal/vaop/ncurrent/full/nchem.1797.html