Main navigation | Main content
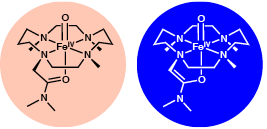
Since 2003, Professor Lawrence Que Jr.’s research group has been investigating the properties of nonheme oxoiron(IV) complexes, which serve as models for high-valent intermediates in the activation of dioxygen by nonheme iron enzymes. Recently, postdoctoral associate Jason England, graduate student Jennifer Bigelow and other members of the Que group, in collaboration with the Eckard Münck group at Carnegie Mellon University, reported in Chemical Science (DOI:10.1039/C3SC52755G) the synthesis and crystallographic characterization of a new oxoiron(IV) complex that shows remarkable thermal stability, despite having an iron(IV) oxidation state. It exhibits a half-life of five days at room temperature, tenfold longer than [FeIV(O)(TMC)(NCMe)]2+, which was the first oxoiron(IV) complex to have been crystallized. The greater stability of the new complex presumably derives from the introduction of the pendant amide ligand, although we do not know precisely what factors increase the lifetime of the complex.
This new complex comes with another twist: the ligand has a CH2 group that is somewhat acidic in the Fe=O complex. Thus treatment of the tan-colored complex with hydroxide turns it into a deeper blue color, which is a color unique among iron(IV)-oxo complexes. Treatment of the blue species with acid returns it to its original tan color. These two species thus represent a conjugate acid/base pair, the first such example for this family of complexes. Besides their colors, the two complexes can be distinguished in solution by their 1H NMR, Mössbauer, resonance Raman, and X-ray absorption spectra and by their electrochemical behavior and reactivity towards cyclohexadiene and triphenylphosphine. Also, the conjugate base is significantly less stable, with a half-life of 90 min at 0 °C.
The ligand has a CH2 group that is somewhat acidic in the Fe=O complex. Thus treatment of the tan-colored complex with hydroxide turns it into a deeper blue color, which is a color unique among iron(IV)-oxo complexes.