Main navigation | Main content
A paper recently published in the Journal of the American Chemical Society and selected for its JACS Spotlights reports the results of the computational study on the capture of uranyl, UO22+ by the super uranyl binding protein (SUP). This study was performed by Post-doctoral Associate Samuel Odoh, Ph.D., and Graduate Student Gary Bondarevsky in Professor Laura Gagliardi’s lab.
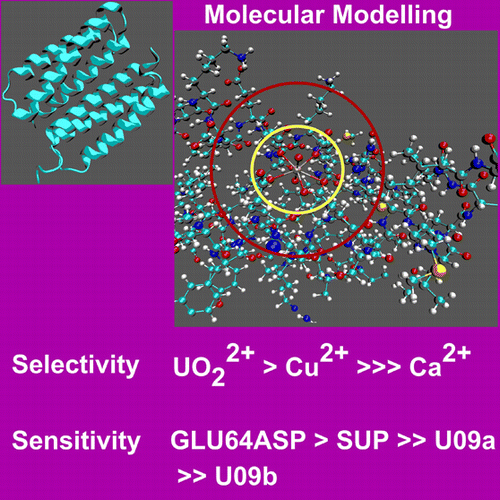
This study shows how UO22+ capture is governed by the nature of the amino acid residues in the binding site, the integrity and strength of the second-sphere hydrogen bond network, and the number of water molecules in the first coordination sphere. Alteration of any of these three factors through mutations generally results in a reduction of the binding free energy of UO22+ to the aqueous protein as well as of the difference between the binding free energies of UO22+ and other ions such as Ca2+, Cu2+, Mg2+, and Zn2+. As part of this, study a mutant of SUP was computationally discovered, specifically the GLU64ASP mutant, that not only binds UO22+ more strongly than SUP, but that is also more selective for UO22+ over other ions.
The predictions from the computations of the Gagliardi’s group were confirmed by experiments performed in the group of Professor Chuan He
at the University of Chicago.
http://pubs.acs.org/doi/abs/10.1021/ja5087563 JACS Spotlights seeks to make published research more accessible to the broader community.