Main navigation | Main content
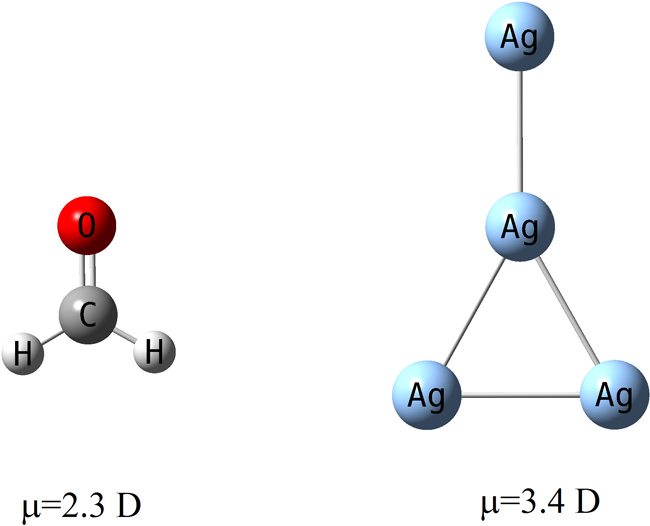
Published research in the Journal of Physical Chemistry by Professor Donald Truhlar and Graduate Student Kaining Duanmu, "Partial Ionic Character Beyond the Pauling Paradigm: Metal Nanoparticle," is featured on page 49 of the Dec. 8 edition of Chemical and Engineering News. They found that metal nanoparticles can have dipole moments much larger than similarly shaped polar organic molecules. Their research showed that homonuclear silver clusters have very uneven charge distributions, whereas the Pauling paradigm would predict that they have even charge distributions, since all the atoms have the same electronegativity. This finding is very important for understanding catalysis.
For example, as shown in the graphic, a Ag4 cluster with the same shape as formaldehyde, has a dipole moments of 3.4 Debyes as compared to 2.3 Debyes for formaldehyde, although formaldehyde is usually considered a polar molecule in organic chemistry, and Ag4 is homonuclear (and hence would have no dipole moment by Pauling’s rules).
This research was sponsored in part by the Inorganometallic Catalyst Design Center.