Main navigation | Main content
Carbon dioxide in the atmosphere from fossil-fuel burning power plants is a problem, contributing to the build-up of greenhouse gases. A possible solution is capturing and isolating the carbon dioxide; however, that is expensive for power plants, and has a downside of substantially increasing the cost of electricity that they generate. Highly efficient adsorbents that can cost-effectively and efficiently remove the carbon dioxide are needed.
Researchers in the University of Minnesota-based Nanoporous Materials Genome Center (NMGC) have made some breakthroughs in their investigation into more efficient gas separation technologies such as those based on advanced solid adsorbents. A joint study between the University of Minnesota and University of California, Berkeley, has shown that diamine-appended metal-organic frameworks can behave as phase-change adsorbents, with unusual step-shaped carbon dioxide adsorption isotherms that shift markedly with temperature.
Results from spectroscopic, diffraction and computational studies show that the origin of the sharp adsorption step is an unprecedented cooperative process in which, above a metal-dependent threshold pressure, carbon dioxide molecules insert into metal-amine bonds, inducing a reorganization of the amines into well-ordered chains of ammonium carbamate.
Working with Professor Laura Gagliardi, graduate student Allison Dzubak, and post-doctoral associates Samuel O. Odoh and Nora Planas performed quantum chemical calculations to determine the structure of the species discussed in this study and their adsorption enthalpies. This study has been featured in Nature. You can also read an article about this research, Materials chemistry: Cooperative carbon capture, by Andrew I. Cooper.
The Nanoporous Materials Genome Center is funded by the Department of Energy. For additional information, go to http://www.chem.umn.edu/nmgc/.
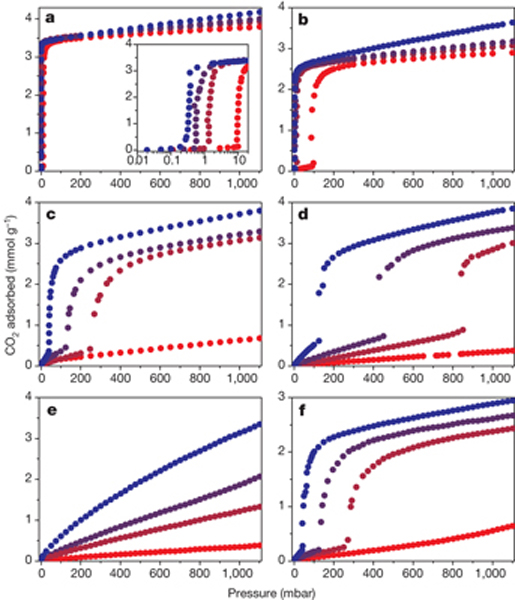
Figure: Carbon dioxide adsorption isotherms at 25 °C (blue), 40 °C (blue-violet), 50 °C (red-violet) and 75 °C (red) for mmen-Mg2(dobpdc) (a), mmen-Mn2(dobpdc) (b), mmen-Fe2(dobpdc) (c), mmen-Co2(dobpdc) (d), mmen-Ni2(dobpdc) (e) and mmen-Zn2(dobpdc) (f). Despite the use of aliphatic amine groups as the CO2 reactive species, the metal-organic framework has an essential role in determining isotherm shape, owing to the importance of metal–ligand reorganization reactions in the mechanism.