Main navigation | Main content
04/29/2016
Recent research from the research group of Professor
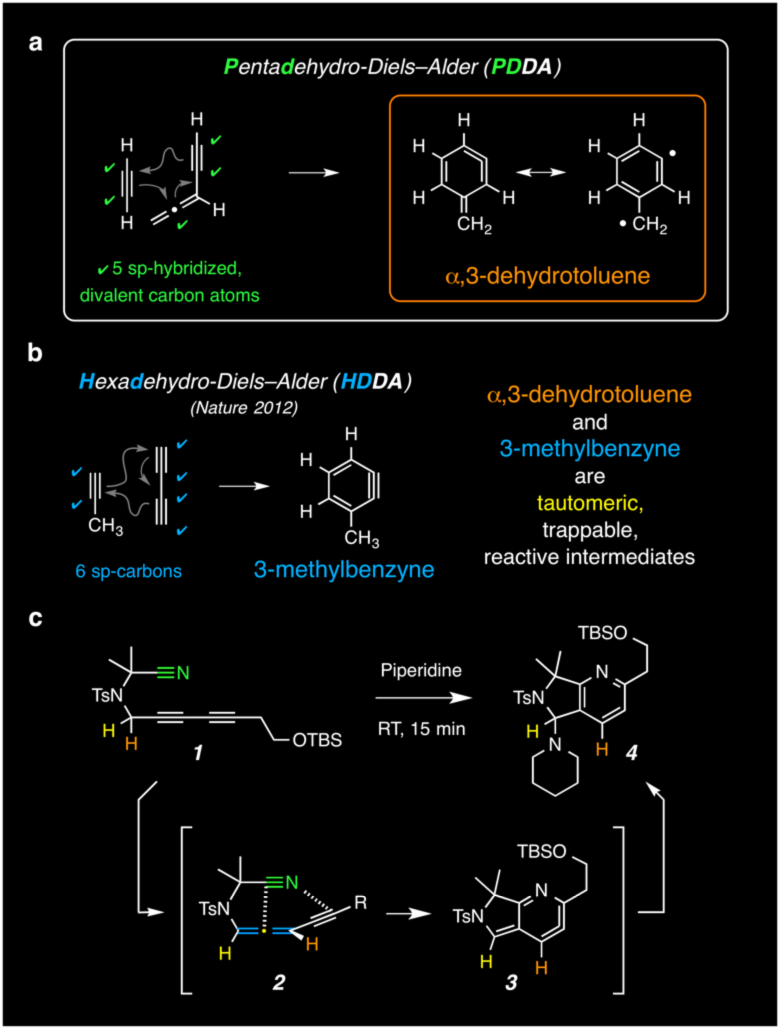
In the April 28 issue of Nature, Teng Wang, Reddy Naredla, and Severin Thompson, researchers in the laboratory of Professor Thomas Hoye, have announced a new variant of a Diels-Alder-like net cycloaddition [Nature 2016, 532, 484–488 (doi:10.1038/nature17429)]. More specifically, they have described the title PDDA reaction in which a (transient) allenyne is captured by a remote alkyne (or nitrile) to produce a highly reactive (and, therefore, further trappable) intermediate known as an α,3-dehydrotoluene. The prototypical version of this process is shown in panel a of the graphic below. This transformation is complementary to the hexadehydro-Diels-Alder (HDDA) reaction [Nature 2012, 490, 208–212 (doi:10.1038/nature11518)], the parent version of which is shown in panel b.
One example of the newly reported reactions is given in panel c. Key features of these PDDA processes are that (i) the allenyne can often be generated by simple tautomerization of a precursor alkyne (see 1 to 2), (ii) the rate of PDDA cyclization of the allenyne (2 to 3) is significantly faster than that of the HDDA reaction of the substrate 1, and (iii) the energetics are such that nitriles now are competent traps for the allenyne, allowing pyridine heterocycles (e.g., 4) to be produced upon trapping of the α,3-dehydrotoluene intermediate (e.g., 3). Nitriles have not been observed to engage 1,3-diynes in HDDA chemistry.