Main navigation | Main content
02/07/2011
Recent research from the research group of Professor Lawrence Que Jr.
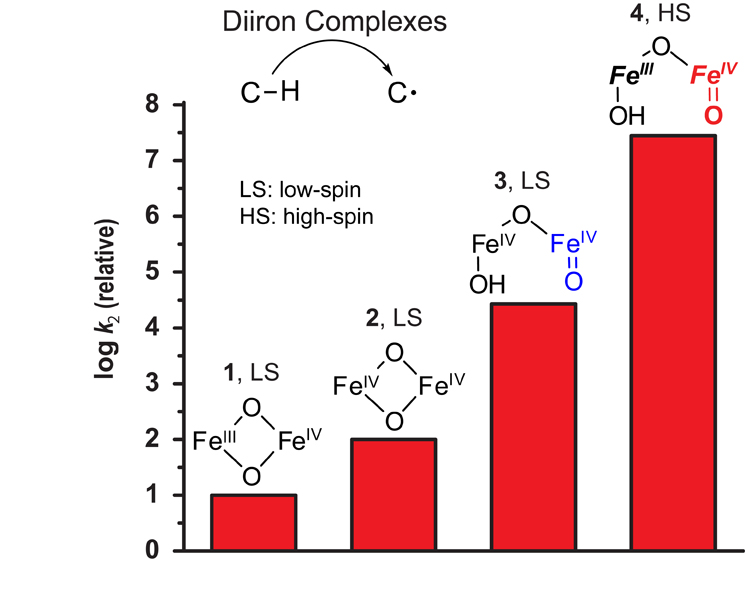
The hydroxylation of methane to methanol in nature is carried out by the nonheme diiron enzyme methane monooxygenase (MMO) found in bacteria called methanotrophs. The fact that this challenging transformation occurs under mild conditions has elicited strong interest in understanding the nature of the oxidant. The nonheme diiron center of MMO activates dioxygen to generate a powerful oxidant called Q, which is postulated to possess a high-valent [FeIV2(µ-O)2] diamond core (2). The synthesis of biomimetic complexes with such diamond cores and the characterization of their C-H bond cleavage reactivity could enhance understanding of the MMO mechanism. Toward this end, Research Associate Gen-Qiang Xue in the laboratory of Professor Lawrence Que Jr. has been investigating the chemistry of a crystallographically characterized complex that has an [FeIIIFeIV(µ-O)2] diamond core (1). As this complex is stable enough to be crystallized, it is not surprising that it is fairly sluggish in reacting with C-H bonds and can cleave only C-H bonds of less than 80 kcal/mol in strength (compared to 104 kcal/mol for methane).
In a paper published in Nature Chemistry 2010, 2: 400, Xue and co-workers reported the conversion of this synthetic complex into a million-fold more reactive species by treatment with hydroxide. Spectroscopic studies showed that the diamond core complex was transformed into a new species with an open [HO-FeIII-O-FeIV=O] core (4). This activity enhancement results from two factors: the formation of a terminal oxoiron(IV) moiety (similar to that of 3) and the conversion of the iron(IV) center from low-spin (S = 1) to high-spin (S = 2). Both factors each enhance C-H bond cleavage reactivity by a thousand-fold. These results suggest that a similar core isomerization might occur in the active site of methane monooxygenase for the cleavage of the methane C-H bond.