Main navigation | Main content
06/30/2011
Recent research from the research group of Professor
A new grant paves the way for Department of Chemistry professors to work with professors at Cambridge University in the United Kingdom.
Professors Laura Gagliardi and Christopher Cramer, co-principal investigators, have received a new National Science Foundation (NSF) International Collaborations in Chemistry (ICC) award of $485,000 for three years. The grant will enable them to engage in research with Professor Jonathan Nitschke from the Department of Chemistry at Cambridge University in the United Kingdom.
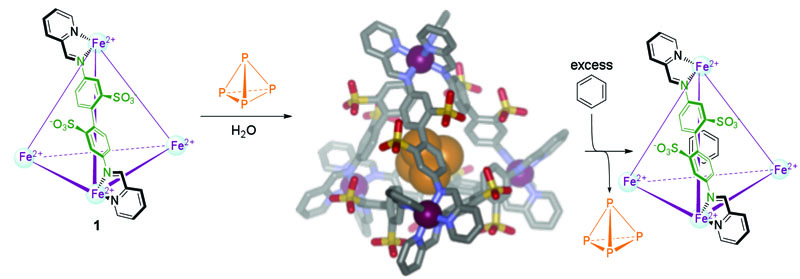
Their research will encompass assessing the fundamental factors affecting binding and recognition in the aqueous host-guest chemistry of small to moderate-sized organic molecules inside self-assembling, metal-templated cages.
By taking advantage of spiral feedback between modeling and experiment, the reseachers will identify key steric and electrostatic interactions in the complexes, the control of which will facilitate the rational design of improved host-guest combinations. Theoretical models based on both quantum and molecular mechanics will be employed. Quantum mechanical models will be employed to supplement experiments with benchmark data for the selection of classical mechanical force fields and also be used to compute spectral data (e.g., nuclear magnetic resonance and ultraviolet-visible spectroscopy) in order to compare to experimental host-guest combinations. Classical mechanical force field modeling will be used to simulate the dynamical behavior of host-guest combinations and for the prediction of potentials of mean force associated with aqueous binding events. With sufficient validating data in hand, in silico design efforts will be undertaken with the goal of focusing synthetic efforts on host-guest combinations showing enhanced selectivity and binding efficiencies.
The knowledge created by this research will shed light on a range of critical phenomena, from how proteins bind substrates within their active sites to how new catalytic transformations might be carried out within purposefully designed hosts.
This interdisciplinary research will provide broad training opportunities and an international research experience for supported junior researchers. In addition, code/software implementing the new models will be made broadly available to the greater scientific community in a variety of well-maintained, licensed software packages.
This research is supported jointly by the NSF Macromolecular, Supramolecular, and Nanochemistry (MSN) Program and the Chemical Theory, Models and Computational Methods Program in the Division of Chemistry.
Figure: Formation of an air-stable, water-soluble complex of white phosphorus (P4) with a self-assembled, metal-templated cage.