Main navigation | Main content
07/26/2011
Recent research from the research group of Professor Donald Truhlar
The research of a team of international researchers, led by Professor Donald Truhlar from the Department of Chemistry and the Minnesota Supercomputing Institute, is featured as one of this week's highlights in Chemical & Engineering (C&EN) News. The article "Grubbs Ruthenium Catalysts Explained," highlights an advanced computational method that unlocks the mystery of reactivity differences between different Grubbs catalysts.
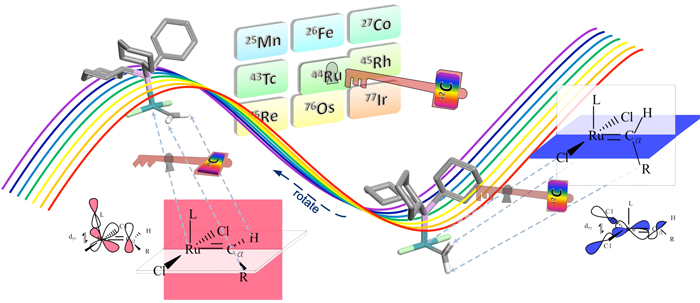
In recent years, a new-generation density functional, called M06-L, has been able to predict the correct ligand effect on the bond energy for real catalysts, and in our paper in Organometallics, it was applied to explore the Grubbs-type metathesis reaction. The question of how ruthenacarbene ligands effect catalytic metathesis, and, in particular, the reason for the slow initiation of the Grubbs-II catalyst and the associated inverse relation between organophosphine dissociation and catalytic activity, have been controversial and are still not fully understood despite numerous experimentsal studies and considerable theoretical effort. For many years, theory was not able to address quantitative issues in catalysis because of the high costs of studying realistic catalysts; calculations in which the catalyst was oversimplified (e.g., replacing organophosphines with unsubstituted phosphines) were untrustworthy because it is known experimentally that such substitutions have a profound effect on the chemistry. Furthermore, reliable electronic wave function methods applicable to small molecules are too expensive to apply to typical real catalysts, and popular density fnctionals have had difficulty providing a balanced view of all the interactions. In the present work, we report a density functional study of realistic Grubbs-type metathesis reaction using the M06-L density functional. A key issue that we examine is the pivotal role of the carbene orientation in the structural evolution accompanying catalysis—its effect on the energy of organophosphine dissociation and the key later steps of olefin binding and ruthenacyclobutane formation.
The results unambiguously show that the energetics and kinetics of rotameric states of ligands are critical for the inverse relation between organophosphine dissociation rate and catalytic activity. The carbene rotamer acts as toggle switch triggering the dissociative mechanism that produces the active catalyst, thereby explaining the long-puzzling differences of first- and second-generation Grubbs catalysts on a unified basis.
Because the slow initiation of Grubbs-II catalysis systems is the major issue impeding progress on further improvement of these Nobel-class catalysts, the researchers believe that their decisive finding, i.e., that the carbene rotamer state explains the slow initiation, will stimulate considerable future work on improved catalyst design. Consideration of the barriers to rotameric state interconversion may open a new venue in understanding the stereochemistry of Grubbs olefin metathesis as required for synthesis of polymers with spatially controlled properties. The researchers are confident that this important result is truly significant and that it will attract a very broad spectrum of interest.
In addition to Truhlar, senior investigators include Professor Hsiao-Ching Yang from the Department of Chemistry at Fu-Jen Catholic University in Taiwan, and Yan Zhao, Ph.D., from the Hewlett-Packard Development Company in Palo Alto, CA. Additional article authors and researchers include Yen-Chin Huang and Professor Tien-Yau Luh from the Department of Chemistry at the National Taiwan University in Taiwan.
Some of these researchers have ties to the University of Minnesota and its Department of Chemistry. Luh was a postdoctoral researcher at the university from 1974 to 1976. Zhao is an alumnus. He was a research associate in Truhlar's group, and earned his doctorate in computational chemistry from the university in 2005.
See the Science and Technology Concentrates in this week's C&EN issue.
Read the complete article at C&EN or Organometallics. The Organometallics article is entitled, "Carbene Rotamer Switching Explains the Reverse Trans Effect in Forming the Grubbs Second-Generation Olefin Metathesis Catalyst."
The article is the lead item of Editors' Choice (page 500) of the July 29 issues of Science magazine, which is published by the American Association for the Advancement of Science. Download the PDF.