Main navigation | Main content
11/11/2011
Recent research from the research group of Professor Philippe Buhlmann
Because HCO3-, CO32-, and CO2 coexist in aqueous solutions, accurate and quick determinations of total CO2 content are required for physiological, industrial, and environmental analysis. A need for measurements in blood plasma arises in clinical chemistry since they are used to detect and monitor electrolyte imbalances. Currently, available sensors suffer from long response times and poor selectivity. A concern is interference from salicylate, which is a metabolite from common headache pills and is found in many clinical samples.
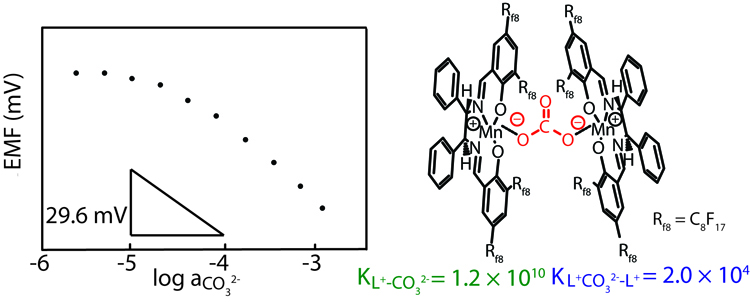
Researchers have developed electrochemical sensors for carbonate that reduce the interference from chloride and salicylate by two and six orders of magnitude, respectively. This breakthrough in carbonate sensing was made possible by the use of fluorous sensing membranes. Because of their extremely low polarity and polarizability, fluorous media are the least polarizable condensed phases known to mankind, and solvate potentially interfering ions poorly. Selectivity for carbonate is achieved by use of fluorophilic manganese(III) salen complexes, developed originally for biphase catalysis by collaborator Gianluca Pozzi from the Istituto di Scienze Tecnologie Molecolari in Milano, Italy. The exceptional selectivity of fluorous membranes doped with these carbonate receptors suggests their use not only for sensing, but also for separations of carbonate from other anions and might even be useful for the energy-efficient sequestration of carbon dioxide.
Preliminary results on these novel carbonate sensors were reported in March 2011 by Research Assistant Li Chen at the Pittsburgh Conference of Analytical Chemistry and was published in the Journal of the American Chemical Society.