Main navigation | Main content
09/11/2012
Recent research from the research group of Professor Jiali Gao
Proton-coupled electron transfer (PCET) reactions are ubiquitous in chemistry and biology, including solar energy conversion, enzyme catalysis and photosynthesis. Formally, the simultaneous migration of an electron and a proton is equivalent to a net hydrogen atom transfer (HAT), but the distinction between a concerted PCET, or CPET, and HAT is rather ambiguous and sometimes controversial. To solve this problem, a multi-configurational multistate density functional theory (MSDFT) was developed in Professor Jiali Gao's group, enabling electron and proton localized Lewis configurations to be constructed from first principles calculations.
In collaboration with Professor Wei Wu and Changwei Wang of Xiamen University in China, Postdoctoral Associate Alessandro Cembran and Research Assistant Makenzie Provorse in the Gao group discovered that the difference between CPET and HAT can be revealed in the third-dimension of a More O’Ferrall-Jencks Diagram.
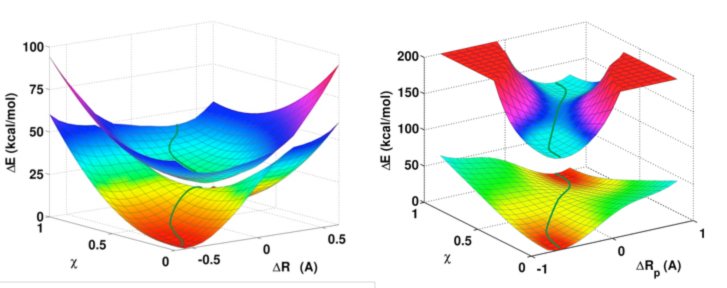
They illustrated this concept in the hydrogen abstraction reactions between C6H5XH and C6H5X* radical, where X = CH2, NH, and O. The reaction between phenol and phenoxy radical is a CPET where a thermally accessible conical intersection seam is seen to separate the reactant and the product regions. The minimum energy paths on the ground and the excited surfaces are nearly orthogonal with weak electronic coupling. On the other hand, the hydrogen abstraction of toluene by benzyl radical is a HAT, in which there is significant overlap between the electronic diabatic states, resulting in large coupling to promote a strongly avoided crossing. In the case of aniline, both CPET and HAT processes are predicted to contribute to the overall kinetics.
Ground and excited state potential energy surfaces as functions of the proton transfer (ΔR) and electronic configuration weight (χ) coordinates for the hydrogen abstraction of phenol by phenoxy radical (left) and toluene by benzyl radical (right). This work is partially supported by the National Institute of Health, extended recently to year 20 through year 23. The article was just published in the Journal of Chemical Theory and Computation, an American Chemical Society publication, and can be read online.