Main navigation | Main content
12/19/2013
Recent research from the research group of Professor
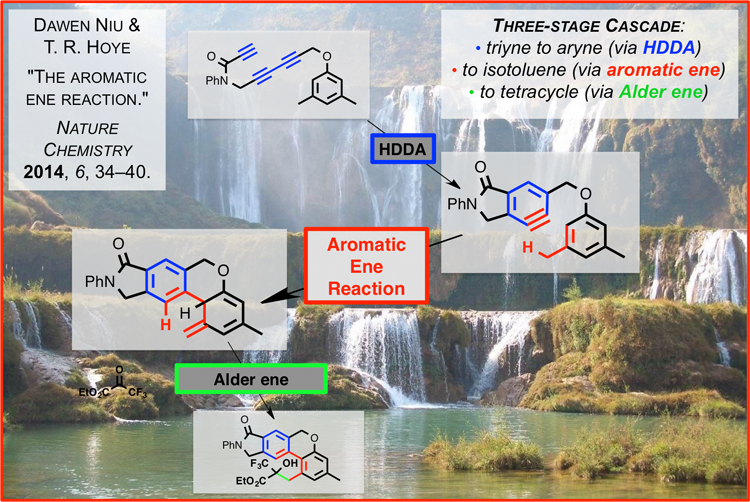
Dawen Niu, working in the laboratory of Professor Thomas Hoye, has discovered the first examples of the title reaction that proceed with high efficiency [Nature Chemistry, January 2014 (doi: 10.1038/nchem.1797)]. They have established that benzyne derivatives formed by the hexadehydro-Diels-Alder (HDDA) reaction [Nature (doi:10.1038/nature11518)] will engage a pendant arene ring in an "ene reaction" fashion. The resulting isotoluene intermediate can be further trapped in productive ways. This energetically favorable "ene-upon-ene cascade reaction involves the overall formation of four carbon–carbon bonds and three rings, requires no external reagents, and generates no by-products."1
1"The aromatic ene reaction," Niu, D.; Hoye, T. R. Nature Chem. 2014, 6, 34–40. http://www.nature.com/nchem/journal/vaop/ncurrent/full/nchem.1797.html