Main navigation | Main content
05/19/2014
Recent research from the research group of Professor
It is increasingly accepted by the scientific, industrial, and political communities that the concentrations of greenhouse gases in the earth’s atmosphere, are increasing. The selective conversion of light alkanes into value-added chemicals is a challenge for society, especially if one considers the recent worldwide increase in natural gas reserves. Nature has an elegant way of performing the activation of C-H bonds by metalloenzymes. A challenge for synthetic and theoretical chemists is to predict this reactivity and duplicate it in new systems.
A collaborative effort between the experimental research group of Jeff Long at the University of California Berkeley and the computational groups of Laura Gagliardi and Don Truhlar at the University of Minnesota has shown significant progress in this direction. Also participating in the study were post-doctoral associate Nora Planas and graduate students Joshua Borycz, Allison L. Dzubak, and Pragya Verma from the University of Minnesota and several students and postdoctoral associates from Berkeley. A paper by these groups that appeared, Sunday May 18, 2014, in Nature Chemistry shows that isolated terminal iron–oxo moieties, supported on a metal–organic framework (MOF), selectively oxidizes ethane into ethanol in the presence of N2O under mild conditions.
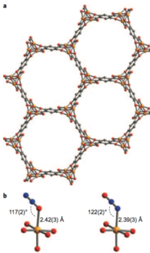
Metal-organic frameworks are also known as coordination polymers. They consist of metal ions coordinated by organic ligands that serve to link the metal centers into a porous three-dimensional network in which the metal ions are available for interactions with adsorbates in the pores. Molecules (such as ethane) entering the pores can undergo catalyzed reactions at the metal centers, which in turn can be designed to have properties not present in other materials. Metal organic frameworks are a major focus of the research carried out by the Nanoporous Materials Genome Center at the University of Minnesota. This center is an Energy Frontier Research Center, which is a national center funded by the U.S. Department of Energy, Office of Basic Energy Sciences, and it is part of the Materials Genome Initiative launched by President Obama in 2011. The work described above shows the great potential of metal organic frameworks for catalytic applications that can promote a more efficient energy economy.
More specifically, this study showed that the metal–organic framework Fe2(dobdc) (where dobdc denotes 2,5-dioxido-1,4-benzenedicarboxylate) and its magnesium-diluted analogue, Fe0.1Mg1.9(dobdc), are able to activate C–H bonds of ethane and thereby convert ethane into ethanol and acetaldehyde using nitrous oxide as the terminal oxidant. Electronic structure calculations indicate that the active oxidant is probably a high-spin S = 5/2 iron(IV)–oxo species.
This study was performed as part of the activities of the Nanoporous Materials Genome Center based at the University of Minnesota supported by the U.S. Department of Energy, Office of Basic Energy Sciences. and was made possible, in part. by the resources of the University of Minnesota Supercomputing Institute.