Main navigation | Main content
06/25/2015
Recent research from the research group of Professor
Professor Truhlar's research paper, “Ultraviolet Absorption Spectrum of Malonaldehyde in Water is Dominated by Solvent-Stabilized Conformations,” has been selected as a Journal of the American Chemical Society Spotlight. This paper was co-written by research associates Xuefei Xu, Ph.D., and Jingjing Zheng. Xu is on the faculty at Tsinghua University in Beijing, and Zheng is a new staff scientist (starting July 1, 2015) at Gaussian Inc. in Wallingford, CT.
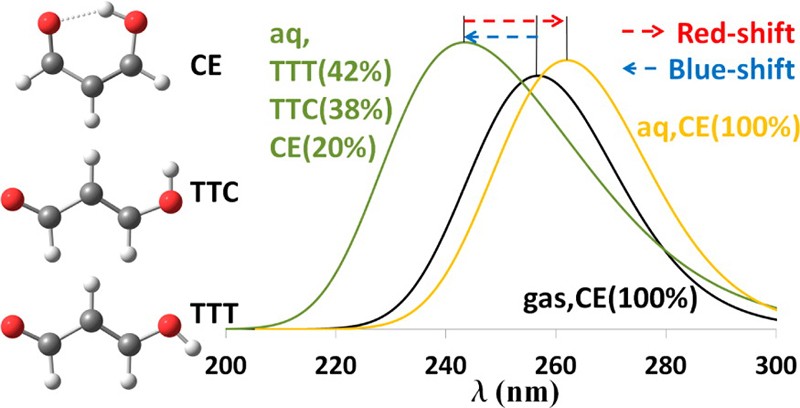
The paper is concerned with the modeling of electronic spectra in solution, which is an important topic across many subareas of chemistry. Not only are spectra of broad fundamental interest in their own right and for analytical purposes, but their assignment is the first step in understanding photochemical processes. The key finding in the paper is that the proper calculation of solvatochromic shifts requires not just the consideration of electrostatic, polarization, and dispersion contributions, which has been standard for many years, but also conformational equilibria, which can change the interpretation not just quantitatively but even qualitatively.
The paper provides a reinterpretation of the spectrum of the prototype molecule malonaldehyde, which is one of the most widely studied molecules in the spectroscopic literature, and yet—because the conformational equilibria were not sorted out—the spectrum and photochemistry have been misinterpreted. The elucidation provided here will change that. Furthermore, the paper shows that quantum chemical analysis has reached a stage where conformational details that are often impossible to glean from experiment can be calculated semiquantitatively and provide essential information necessary for a proper interpretation of experimental data. Quantitatively, the paper also shows that even for a single conformer, ensemble averaging of excitation energies and oscillator strengths can have a significant effect on the location of the band maxima, with a shift of 0.33 eV being found.
“Ultraviolet Absorption Spectrum of Malonaldehyde in Water is Dominated by Solvent-Stabilized Conformations,” X. Xu, J. Zheng, and D. G. Truhlar, Journal of the American Chemical Society, online as Article ASAP. dx.doi.org/10.1021/jacs.5b04845