Main navigation | Main content
11/03/2015
Recent research from the research group of Professor
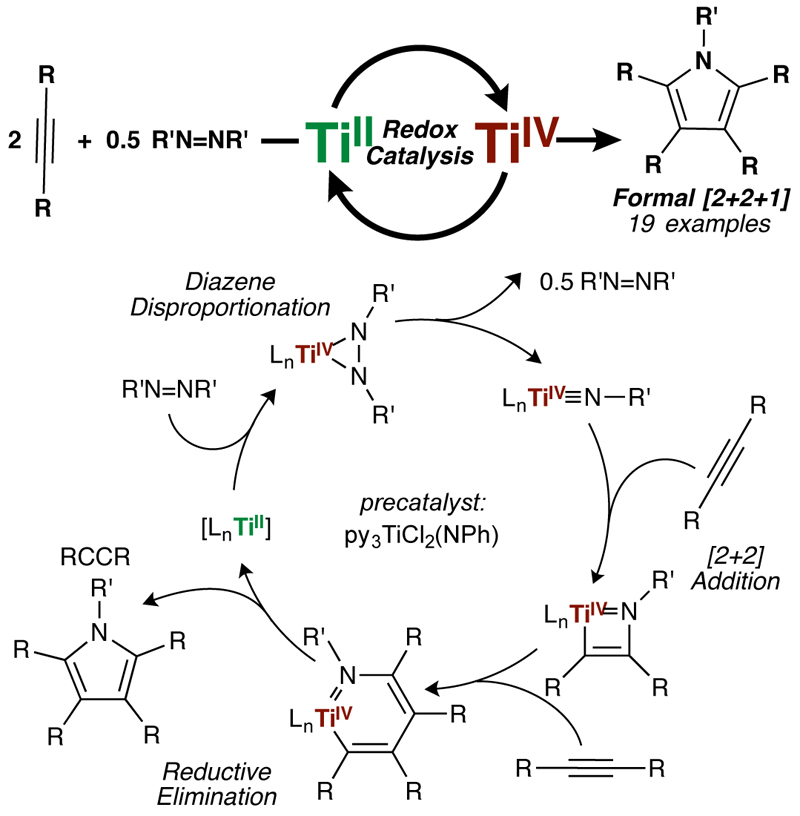
In a study published in Nature Chemistry, graduate student Zach Gilbert, post-doctoral scholar Ryan Hue, and Professor Ian Tonks have shown that simple Ti catalysts are capable of oxidatively coupling alkynes with diazenes to generate polysubstituted pyrroles.
Early transition metals such as titanium are attractive targets for catalysts: they are earth-abundant and typically have low toxicity when compared to noble metals such as palladium or platinum. However, utilizing early metals for catalytic reactions that require oxidation state changes is challenging because of the extreme thermodynamic stability of their high oxidation states (Ti, for example, predominantly exists at Ti4+).
In their study, the research team found that when simple Ti imido precatalysts were reacted with alkynes and diazenes, pyrroles were formed catalytically. The mechanism of the reaction proceeds through a unique Ti2+/Ti4+ oxidation-reduction cycle, where the key turnover step is reoxidation of the low-valent Ti2+ by the diazene. The team hypothesizes that diazenes are privileged oxidants for early transition metals, and that they hold the key to various early metal catalyzed oxidative nitrene transfer reactions.
The Tonks group is currently exploring the detailed mechanism of this reaction. Researchers hope to develop their method into a general and practical route to make polysubstituted pyrroles, which are notoriously challenging synthetic targets found in many bioactive natural products and pharmaceuticals such as the cholesterol-lowering statin Lipitor.
Their full report can be found at http://dx.doi.org/10.1038/nchem.2386.