Return to: U of M Home

Center Information
Metalloprotein Interest Group


Recent Publications
Que Group:
- England, J.; Bigelow, J. O.; Van Heuvelen, K. M.; Farquhar, E. R.; Martinho, M.; Meier, K. K.; Frisch, J. R.; Münck, E.; Que, L., Jr., An Ultra-stable Oxoiron(IV) Complex and Its Blue Conjugate Base Chem. Sci. 2014, 5, 1204-1215 (DOI: 10.1039/C3SC52755G).
- Oloo, W.N.; Meier, K. K.; Wang, Y.; Shaik, S.; Münck, E.; Que, L., Jr., Identification of a Low-spin Acylperoxoiron(III) Intermediate in Bio-inspired Nonheme Iron-Catalyzed Oxidations Nature Communications 2014, DOI: 10.1038/ncomms4046.
- Fielding, A. J.; Lipscomb, J. D.; Que, L., Jr., A two-electron shell game: intermediates of the extradiol cleaving catechol dioxygenases J. Biol. Inorg. Chem. 2014, 19, 491-504 (DOI: 10.1007/s00775-014-1122-9).
- Chiang, C.-W.; Kleespies, S. T.; Stout, H. D.; Meier, K. K.; Li, P.-Y.; Bominaar, E. L.; Que, L., Jr.; Münck, E.; Lee, W.-Z., Characterization of a Paramagnetic Mononuclear Nonheme Iron-Superoxo Complex J. Am. Chem. Soc. 2014, 136, 10846–10849 (DOI: 10.1021/ja504410s).
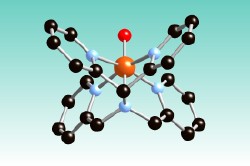
- McDonald, A. R.; Que, L., Jr. "High-Valent Nonheme Iron-Oxo Complexes: Synthesis, Structure, and Spectroscopy. Coord." Chem. Rev. 2013, 257, 414-428(10.1016/j.ccr.2012.08.002).
Lipscomb Group:
- Kovaleva, E.G.; Lipscomb, J.D., “Crystal Structures of Fe2+ Dioxygenase Superoxo, Alkylperoxo, and Bound Product Intermediates.” Science 2007, 316, 453-457.
- Chakrabarty, S.; Austin, R.N.; Deng, D.; Groves, J.T.; Lipscomb, J D., “Radical Intermediates in Monooxygenase Reactions of Rieske Dioxygenases.” J. Am. Chem. Soc. 2007, 129, 3514-3515.
- Neibergall, M.B.; Stubna, A.; Mekmouche, Y.; Münck, E.; Lipscomb, J D., “Hydrogen Peroxide Dependent CIS-dihydroxylation of Benzoate by Fully Oxidized Benzoate 1,2-dioxygenase.” Biochemistry 2007, 46, 8004-8016.
- Zhang, J.; Wallar, B.J.; Popescu, C.V.; Renner, D.B.; Thomas, D.D.; Lipscomb, J.D., “Methane Monooxygenase Hydroxylase and B Component Interactions.” Biochemistry 2006, 45, 2913-2926.
- Zheng, H.; Lipscomb, J.D., “Regulation of Methane Monooxygenase Catalysis Based on Size Exclusion and Quantum Tunneling.” Biochemistry 2006, 45, 1685-1692.
Tolman Group:
- Synthetic Models of Copper-Nitrosyl Species Proposed as Intermediates in Biological Denitrification. Debra J. Salmon and William B. Tolman, Struct. Bond. 2014, 154, 137-154.
- Reactivity of (Dicarboxamide)M(II)-OH (M = Cu, Ni) Complexes: Reaction with Acetonitrile to Yield M(II)-Cyanomethides. Jacqui Tehranchi, Patrick J. Donoghue, Christopher J. Cramer, and William B. Tolman, Eur. J. Inorg. Chem. 2013, 4077-4084.
- Isolation of a 2-Hydroxytetrahydrofuran Complex from Copper-Promoted Hydroxylation of THF. Mohammad Reza Halvagar and William B. Tolman, Inorganic Chemistry, 2013, 52, 8306-8308.
- Linkage Isomerism in Transition Metal Complexes of Mixed (Arylcarboxamido)(arylimino)pyridine Ligands. David W. Boyce, Debra J. Salmon, and William B. Tolman, Inorg. Chem. 2014, 53, 5788–5796.
- Oxygen-Bridged Dicopper(II,III) and (III,III) Complexes: Models for Putative Intermediates in Oxidation Catalysis. Mohammad Reza Halvagar, Pavlo V. Solntsev, Hyeongtaek Lim, Britt Hedman, Keith O. Hodgson, Edward I. Solomon, Christopher J. Cramer, and William B. Tolman, J. Am. Chem. Soc. 2014, 136, 7269-7272.
Wilmot Group:
- Cedervall, P., Hooper, A.B. & Wilmot, C.M. (2013) Structural studies of hydroxylamine oxidoreductase reveal a unique heme cofactor and a previously unidentified interaction partner. Biochemistry 52: 6211-6218.
- Johnson, B.J., Yukl, E.T., Klema, V.J., Klinman, J.P. & Wilmot, C.M. (2013) Structural snapshots from the oxidative half-reaction of a copper amine oxidase: implications for O2 activation. J. Biol. Chem. 288: 28409-28417.
- Klema, V.J., Solheid, C.J., Klinman, J.P. & Wilmot, C.M. (2013) Structural analysis of aliphatic vs. aromatic substrate specificity in a copper amine oxidase from Hansenula polymorpha. Biochemistry 52: 2291-2301.
- Yukl, E.T., Liu, F., Krzystek, J., Shin, S., Jensen, L.M.R., Davidson, V.L., Wilmot, C.M. & Liu, A. (2013) A di-radical intermediate within the context of tryptophan tryptophylquinone biosynthesis. Proc. Natl. Acad. Sci. USA. 110: 4569-4573.
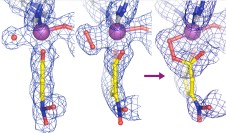
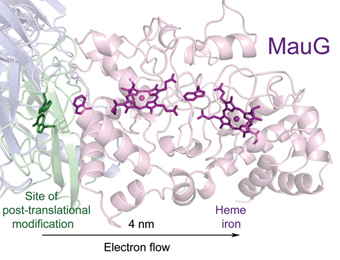
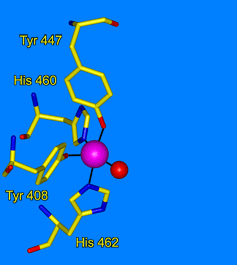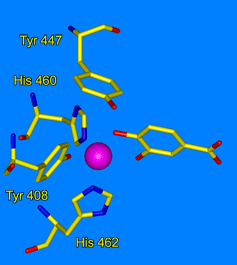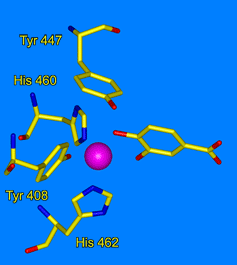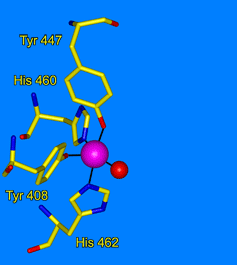
- Jensen, L.M.R., Meharenna, Y.T., Davidson, V.L., Poulos, T.L., Hedman, B., Wilmot, C.M. & Sarangi, R. (2012) Geometric and electronic structures of the His-Fe(IV)=O and His-Fe(IV)-Tyr heme of MauG. J. Biol. Inorg. Chem. 17:1241-1255.
Ohlendorf Group:
- Brown, C. K., M. W. Vetting, C. A. Earhart, and D. H. Ohlendorf, "Biophysical analyses of designed and selected mutants of protocatechuate 3,4-dioxygenase." Annu. Rev. Microbiol. 2004, 58, 555-585.
- Earhart, C. A., M. W. Vetting, R. Gosu, I. Michaud-Soret, L. J. Que, and D. H. Ohlendorf, "Structure of catechol 1,2-dioxygenase from Pseudomonas arvilla." Biochem. Biophys. Res. Commun. 2005, 338, 198-205.
- Valley, M. P., C. K. Brown, D. Burk, N. Elango, M. W. Vetting, D. H. Ohlendorf, and J. D. Lipscomb, "Roles of equatorial tyrosyl iron ligand of protocatechuate 3,4-dioxygenase in catalysis." Biochemistry, 2005, 44, 11024-11039.
- Vetting, M. W., D. A. D'Argenio, L. N. Ornston, and D. H. Ohlendorf, "Structure of Acinetobacter calcoaceticus protocatechuate 3,4-dioxygenase at 2.2 Å resolution: Implications for the mechanism of an intradiol dioxygenase." Biochemistry, 2000, 39, 7943-7955.
- Vetting, M. W., and D. H. Ohlendorf, "The 1.8 Å crystal structure of catechol 1,2-dioxygenase reveals a novel hydrophobic helical zipper as a subunit linker." Structure Fold. Des. 2000, 8, 429-440.
Pierre Group:
- Peterson, K. L.; Dang, J. L; Weitz, E. A.; Lewandowski, C.; Pierre, V. C.* “Effect of lanthanide complex structure on cell viability and association” Inorg. Chem. 2014, 53, 6013-6021.
- Pierre, V. C.*; Allen, M. J.*; Caravan, P.* “Contrast Agents for Magnetic Resonance Imaging: 30+ Years and Where are We Going?” J. Biol. Inorg. Chem. 2014, 19, 127-131.
- Weitz, E. A.; Chang, J.; Rosenfield, A. H.; Morrow, E.; Pierre, V. C.* “The Basis for the Molecular Recognition and the Selective Time-Gated Luminescent Detection of ATP and GTP by a Lanthanide Complex” Chem. Science 2013, 4, 4052-4060.
- Peterson, K. L.; Margherio, M.; Doan, P.; Wilke, K. T.; Pierre, V. C. “The Basis for Sensitive and Selective Time-Delayed Luminescence Detection of Hydroxyl Radical by Lanthanide Complexes” Inorg. Chem. 2013, 52, 9390-9098.
- Weitz, E. A.; Lewandowski, C.; Smolensky, E. D.; Marjanska, M.; Pierre, V. C. “A Magnetoplasmonic Imaging Agent for Copper(I) with dual Response by MRI and Dark Field Microscopy” ACS Nano 2013, 7, 5842-5849.
- Smolensky, E. D.; Peterson, K. L.; Weitz, E. A.; Lewandowski, C.; Pierre, V. C.* “Magnetoluminescent Light-Switches: Dual Modality in DNA Detection” J. Am. Chem. Soc. 2013, 135, 8966-8972.
- Smolensky, E. D.; Park, H.-Y. E.; Zhou, Y.; Rolla, G. A.; Marjanska, M.; Botta, M.; Pierre, V. C.* “Scaling Laws at the Nano Size: the Effect of Particle Size and Shape on the Magnetism and Relaxivity of Iron Oxide Nanoparticle Contrast Agents” J. Mat. Chem. B 2013, 1, 2818-2828.
- Weitz, E. A.; Chang, J. Y.; Rosenfield, A.; Pierre, V. C.* “A Selective Luminescent Sensor for the Time-Gated Detection of ATP” J. Am. Chem. Soc. 2012, 134, 16099-16102.
- Smolensky, E. D.; Marjanska, M.; Pierre, V. C.* “A Responsive Particulate MRI Contrast Agent for Copper(I): a Cautionary Tale” Dalton Trans. 2012, 41, 8039-8046. Invited contribution – special issue on New Talent in the Americas
- Smolensky, E. D.; Zhou, Y.; Pierre, V. C.* “Magnetoluminescent Agents for Dual MRI and Time-Gated Fluorescence Imaging” –Eur. J. Inorg. Chem. 2012, 12, 2141-2147. Invited contribution – special issue on Metal-Based MRI probes. Featured in ChemistryView
- Smolensky, E. D.; Neary, M. C.; Zhou, Y.; Berquo, T. S.; Pierre, V. C.* “Fe3O4@organic@Au: High Saturation Magnetization Core-Shell Nanocomposites as Magnetoplasmonic MRI Contrast Agents” Chem. Commun. 2011, 47, 2149-2151.
- Weitz, E. A.; Pierre, V. C.* “A Ratiometric Probe for the Time-Gated Luminescence Detection of Potassium in Water” Chem. Commun. 2011, 47, 541-543. Invited contribution – special issue on Emerging Investigators.
- Smolensky, E. D.; Park, H.-Y. E.; Berquo, T. S.; Pierre, V. C.* “Surface Functionalization of Iron Oxide Nanoparticles for MRI Applications: Effect of Anchoring Group and Ligand Exchange Protocol” Contrast Media Mol. Imaging, 2011, 6, 189-199.
Hooper Group:
-
Basumallick, L.; Sarangi, R.; Elmore, B.; Hooper, A.B.; Solomon, E.I. "Spectroscopic and Density Functional Studies of the Red Copper Site in Nitrosocyanin: Role of the Protein in Active site Geometric and Electronic Structure," J. Am. Chem. Soc.2005, 127, 3531-3544.
- Pulcu, G.S.; Elmore, B.L.; Arciero, D.M.; Hooper, A.B.; Elliot, S.J. "Direct electrochemistry of tetraheme cytochrome c554 from Nitrosomonas europaea: redox cooperativity and conformational gating." J. Am. Chem. Soc. 2007, 129, 1838-1839.
- Pearson, A.R.; Elmore, B.O.; Yang, C.; Ferrara, J.D.; Hooper, A.B.; Wilmot, C.M. "The crystal structure of cytochrome P460 of Nitrosomonas europea reveals a novel cytochrome fold and heme-protein cross-link." Biochemistry, 2007, 46, 8340-8349.
- Kim, H. J.; Zatsman, A.; Upadhyay, A. K.; Whittaker, M.W.; Bergmann, D.J.; Hendrich, M.P.; Hooper, A.B. " Membrane Tetraheme Cytochrome cm552 of the Ammonia-Oxidizing Nitrosomonas europaea; a Ubiquinone Reductase." Biochemistry, 2008, 47, 6539-6551.
- Klotz, M.G.; Schmid, M.C.; Strous, M.; op den Camp, H.J.M.; Jetten, M.S.M.; Hooper, A.B. " Evolution of an octaheme cytochrome c protein family that is key to aerobic and anaerobic ammonia oxidation by bacteri.", Environmental Microbiology, 2008, 10, 3150-3163.
Lu Group:
- Tereniak, S. J.; Carlson, R. K; Clouston, L. J.; Young, V. G., Jr.; Bill, E.*; Maurice, R.; Cheng, Y.-S.;
Kim, H. J.; Gagliardi, L.*; Lu. C. C.* “Role of the Metal in the Bonding and Properties of Bimetallic Complexes with Metal-Metal Interactions Involving Manganese, Iron, and Cobalt.” J. Am. Chem. Soc. 2014, 136, 1842-1855. http://dx.doi.org/10.1021/ja409016w
- Clouston, L. J.; Siedschlag, R. B.; Rudd, P. A.; Planas, N.; Hu, S.; Miller, A. D.; Gagliardi, L.; Lu, C. C.* “Systematic Variation of Metal-Metal Bond Order in Metal-Chromium Complexes” J. Am. Chem. Soc. 2013, 135, 13142-13148. http://dx.doi.org/10.1021/ja406506m
- Zall, C. M.; Clouston, L. J.; Young, V. G., Jr.; Ding, K.; Kim, H. J.; Zherebetsky, D.; Cheng, Y.-S.; Bill, E.*; Gagliardi, L.*; Lu, C. C.* “Mixed-Valent Dicobalt and Iron-Cobalt Complexes with High-Spin Configurations and Short Metal-Metal Bonds.” Inorg. Chem. 2013, 52, 9216-9228.
http://dx.doi.org/10.1021/ic400292g
- Rudd, P. A.; Planas, N.; Bill, E.; Gagliardi, L.; Lu, C. C.* “Dinitrogen Activation at Iron and Cobalt Metallalumatranes.” Eur. J. Inorg. Chem. 2013, 2013, 3898-3906.
http://dx.doi.org/10.1002/ejic.201300272
- Rudd, P. A.; Liu, S.; Planas, N.; Bill, E.; Gagliardi, L.*; Lu, C. C.* “Multiple Metal-Metal Bonds in Iron-Chromium
Complexes.” Angew. Chem. Int. Ed. Engl. 2013, 52, 4449-4452.
http://dx.doi.org/10.1002/anie.201208686

News Items